
For forensic toxicology use only.
This application brief describes a robust UPLC-MS/MS method for the analysis of cTHC in hair that can routinely meet the guidelines for the confirmation cut-off concentration recommended by the Society of Hair Testing (SoHT).1
Robust and sensitive UPLC-MS/MS method for the determination of cTHC in hair.
The use of hair as a biological matrix for forensic toxicology testing has increased in popularity over the last decade. In contrast to traditional matrices, such as blood and urine, hair offers an extended detection window and can be used to provide a chronological history of drug exposure over several months to years if segmental analysis is performed. Human hair is known to grow at approximately 1 centimeter per month. Drugs can be incorporated into the hair by several mechanisms including, passive diffusion from the blood supply at the follicle into the growing hair matrix; diffusion into the hair shaft from sweat or sebum, and through external contamination such as smoke or contaminated hands.
Hair collection is a non-invasive technique and can be achieved without the privacy and adulteration issues associated with urine collection and, in contrast to blood samples, hair does not require medically trained staff to collect the sample. Furthermore, hair samples can be easily stored.
Cannabis is the most widely used controlled substance in the world and long-term use can lead to dependency. Cannabinoids are one of the most commonly detected classes of drugs; consequently their analysis is of key importance in forensic testing. Delta-9-tetrahydrocannabinol (THC) is the major psychoactive element present in the plant Cannabis sativa and produces a number of metabolites including 11-nor-9-carboxy-Δ9- tetrahydrocannabinol (cTHC).
To differentiate between actual cannabis intake from passive environmental cannabis smoke exposure, the SoHT requires that the positive identification of THC in hair samples must be confirmed by measuring the endogenous metabolite cTHC. However, analysis of this metabolite is very challenging, as it is typically found at low pg/mg concentrations and the amount of sample available is often very limited, thus high sensitivity analytical techniques are required.
Control hair was collected from volunteers and following successive decontamination with dichloromethane, methanol, and diethyl ether it was scissor minced into 1 to 2 mm segments. The minced hair was stored at 4 °C until required. M3 Reagent was supplied by Comedical, Trento, Italy. http://www.comedical.biz/
Control hair (20 mg) was weighed into a centrifuge tube with a sealed cap and spiked with cTHC at concentrations ranging from 0 to 10 pg/mg. Internal standard (80 pg of cTHC-d3) was added along with M3 Reagent. The samples were incubated for 60 min at 100 °C in an incubator and once cooled the entire sample was loaded onto an OASIS PRiME HLB 30 mg Cartridge (p/n 186008055). The sample was washed with an acetonitrile solution followed by hexane. The cTHC was eluted with acetonitrile/ methanol (9:1 v/v) and following evaporation of the solvent, the samples were reconstituted in a methanol solution and transferred to Waters Total Recovery Vials.
The ACQUITY UPLC I-Class (FTN) System was fitted with a 30 μL needle, which allowed 15 μL of sample to be analyzed; cTHC was separated using a gradient of ammonium fluoride pH 9.5 and methanol on a BEH C18 Column. Two MRM transitions for cTHC were monitored using the Xevo TQ-S micro Mass Spectrometer i.e., m/z 343 > 191 (quantifier), and m/z 343 > 245 (qualifier). The internal standard (cTHC-d3) was monitored using the transition m/z 346 > 194.
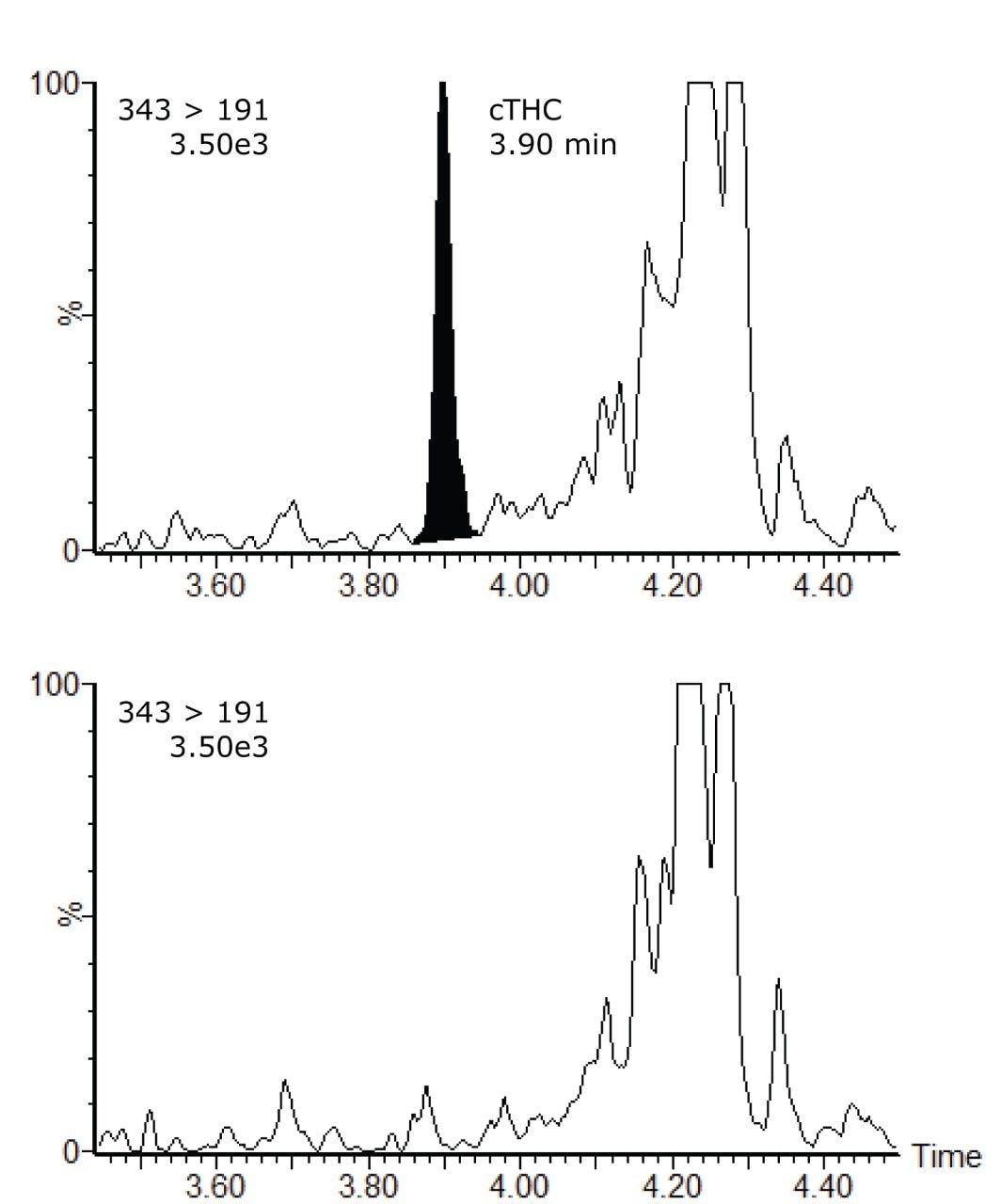
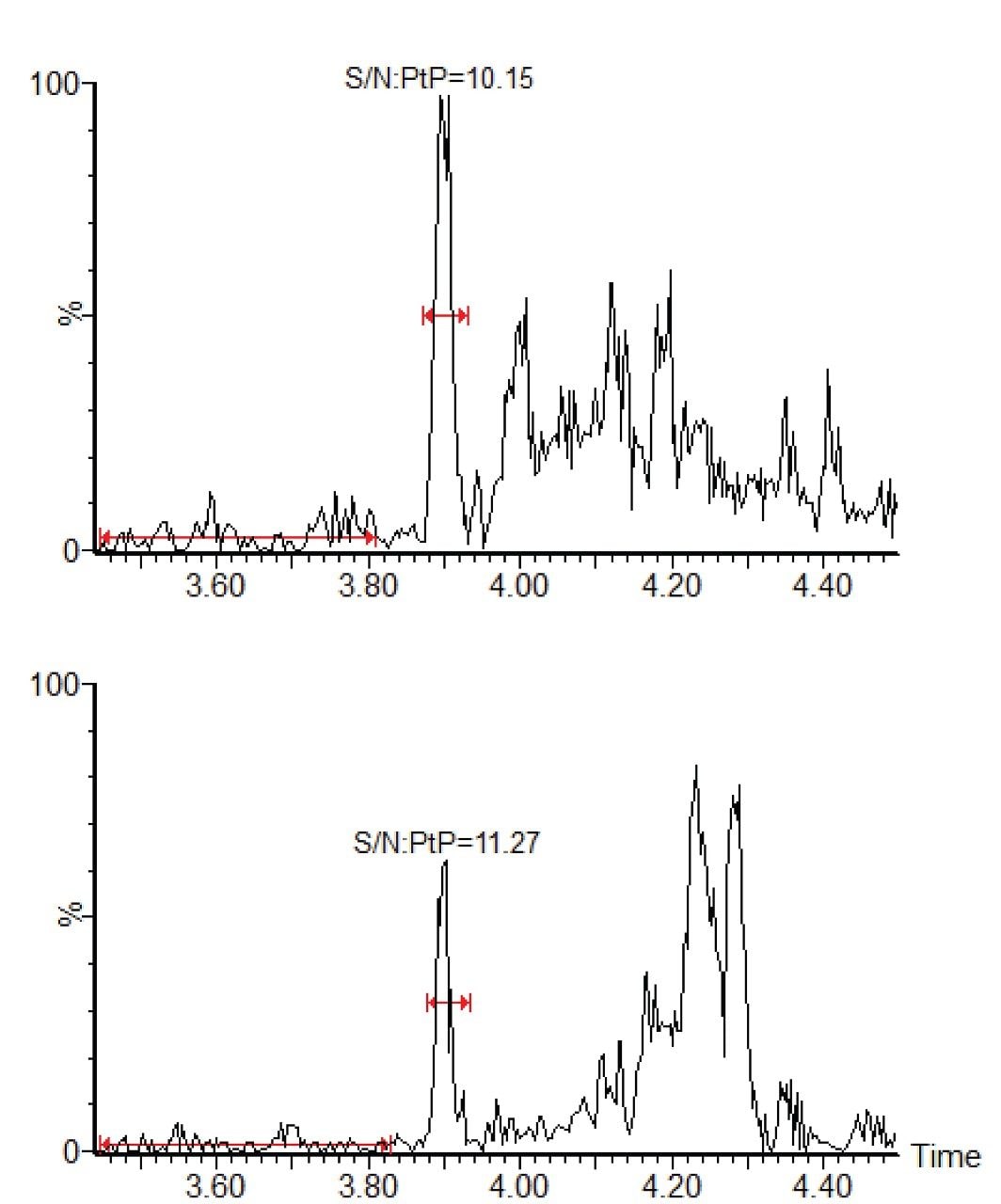
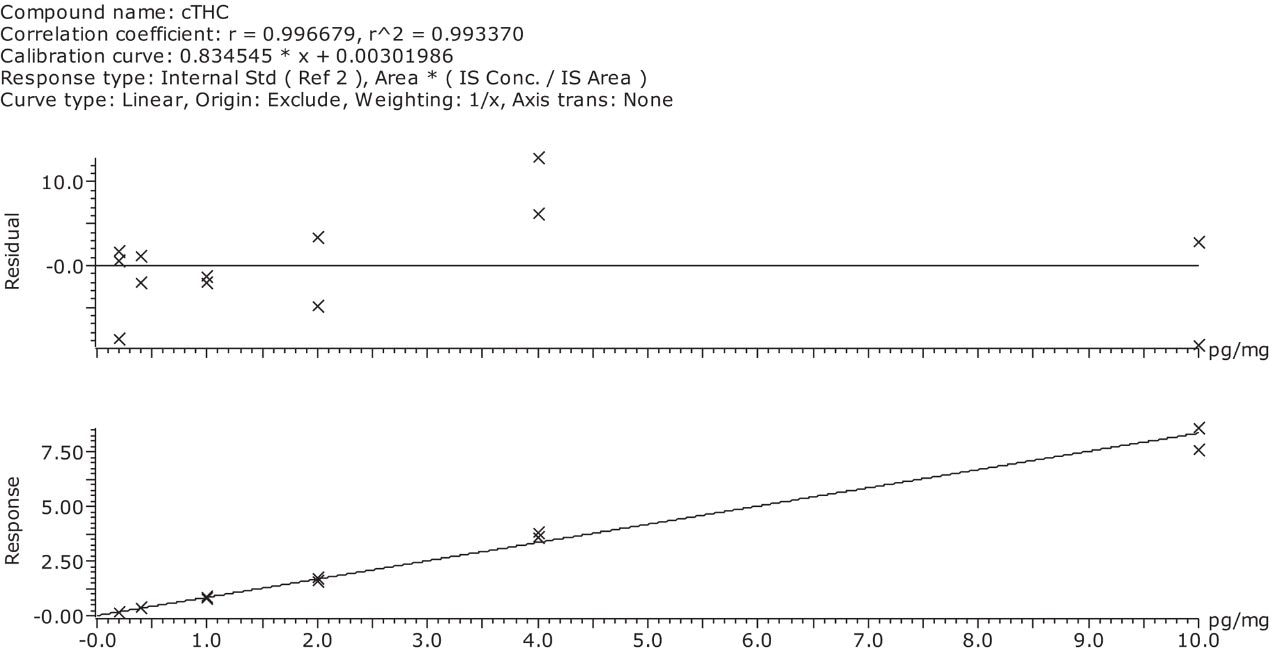
A comparison of a control (blank) hair extract with a spiked hair extract is shown in Figure 1. The figure shows the smoothed and integrated quantifier MRM trace for both samples. The concentration of the spiked sample is 0.2 pg/mg, which is the confirmation cut-off concentration recommended by the SoHT. The signal to noise calculations (peak to peak) for the quantifier and qualifier MRM transitions from a 0.2 pg/mg cTHC spiked hair sample are shown in Figure 2. The linearity of the assay was investigated over the range 0 to 10 pg/mg and the calibration curve along with the residuals plot are shown in Figure 3.
The rise of forensic toxicology testing has highlighted the need for a quick, accurate, reliable, and robust method to quantify compounds in various biological matrices. The use of hair allows for simple, supervised, and non-invasive collection of a matrix which contains analytes commonly measured in such testing schemes.
The ACQUITY UPLC I-Class/Xevo TQ-S micro System has demonstrated the required analytical sensitivity to detect cTHC in hair at sub pg/mg concentrations.
720006363, December 2018