
For research use only. Not for use in diagnostic procedures.
A rapid UPLC-MS/MS methodology has been developed for the analysis of tryptophan and seven of its metabolites in various matrices. This method utilizes a generic, flexible LC-MS platform that can be used for various compound classes (including metabolomics, lipidomics, and proteomics), meaning it can be applied as part of a suite of analyses that are run sequentially as part of a targeted multi-omics workflow.
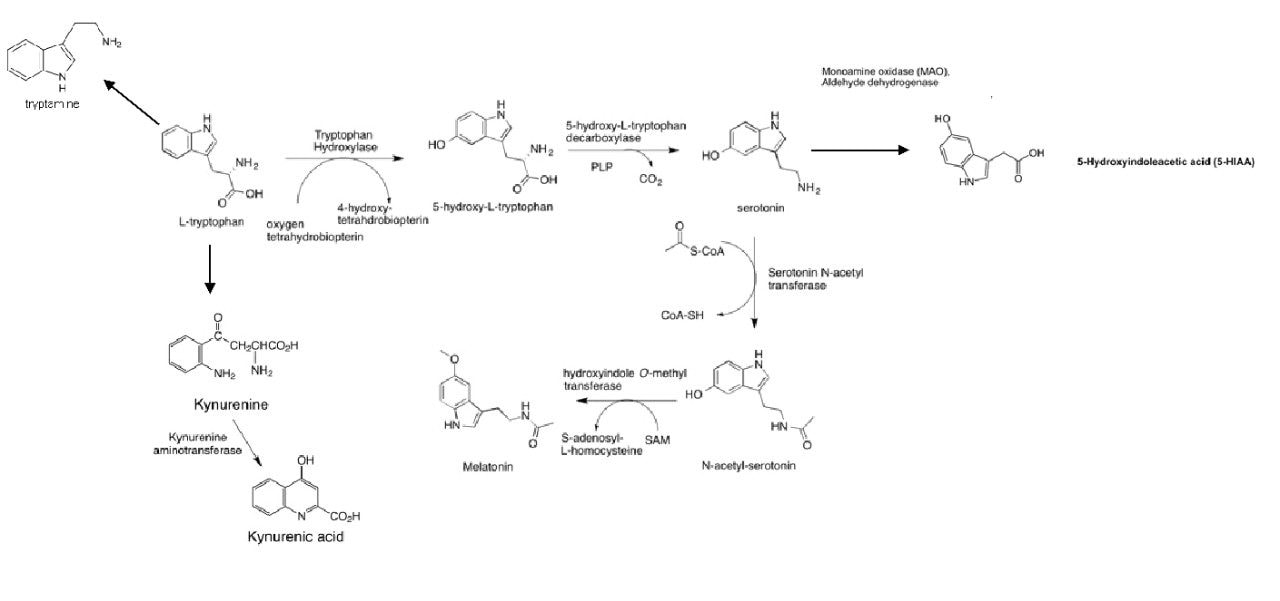
Tryptophan is an amino acid that, along with its metabolites (Figure 1), plays a crucial role in a variety of biological functions, including brain health andcardiometabolic regulation. As such, the study of tryptophan metabolism is of interest in biomedical research, and a high-throughput analytical method for the analysis of tryptophan and its metabolites enables the analysis of large cohort studies. Presented here is a high-throughput UPLC method that utilizes a flexible LC-MS platform. The method can be used seamlessly alongside methods for other compound classes contained in the Targeted Omics Method Library and enables labs to easily and conveniently increase analyte coverage without the burden of method development.
One hundred microliters of human urine were diluted 1:10 with 900 μL of LC-MS-grade water. One microliter of this was then injected onto the UPLC-MS/MS system.
One hundred fifty microliters of human plasma were extracted using solid-phase extraction (SPE). Prior to extraction, the samples were centrifuged so that they would not block the SPE plate. A 150-μL aliquot of each sample was then diluted with 450 μL of water before loading onto an Oasis HLB PRiME μElution Plate. The plate was then washed with 150 μL of water. The analytes were eluted from the SPE plate using 25 μL of methanol. The eluate was then diluted 1:1 with 25 μL of water before injecting 1 μL onto the UPLC-MS/MS system.
UPLC separation was performed with an ACQUITY UPLC I-Class System (Fixed Loop), equipped with a CORTECS T3, 2.7 μm (2.1 × 30 mm) Analytical Column. One microliter of sample was loaded onto the column and eluted under gradient conditions at a flow rate of 0.45 mL/min. Mobile phase A was 0.01% formic acid (aq) and mobile phase B was 50% isopropanol in acetonitrile containing 0.01% formic acid. After an initial 0.75-minute hold at 0% mobile phase B, tryptophan and its associated metabolites were eluted from the column and separated with a gradient of 0–70% mobile phase B over 0.95 minutes, followed by a one-minute column wash at 98% mobile phase B. The column was then re-equilibrated to initial conditions. The analytical column temperature was maintained at 60 °C.
The analytes were detected using multiple reaction monitoring (MRM) analyses using a Xevo TQ-S micro Mass Spectrometer. All experiments were performed in positive electrospray ionization (ESI+) mode. The ion source temperature and capillary voltage were kept constant and set to 150 °C and 2.0 kV, respectively. The cone gas flow rate was 50 L/hr and desolvation temperature was 650 °C.
Method information was imported onto the LC-MS system using the Quanpedia functionality within MassLynx. This extendable and searchable database produces LC and MS methods, as well as processing methods, for use in TargetLynx for compound quantification.
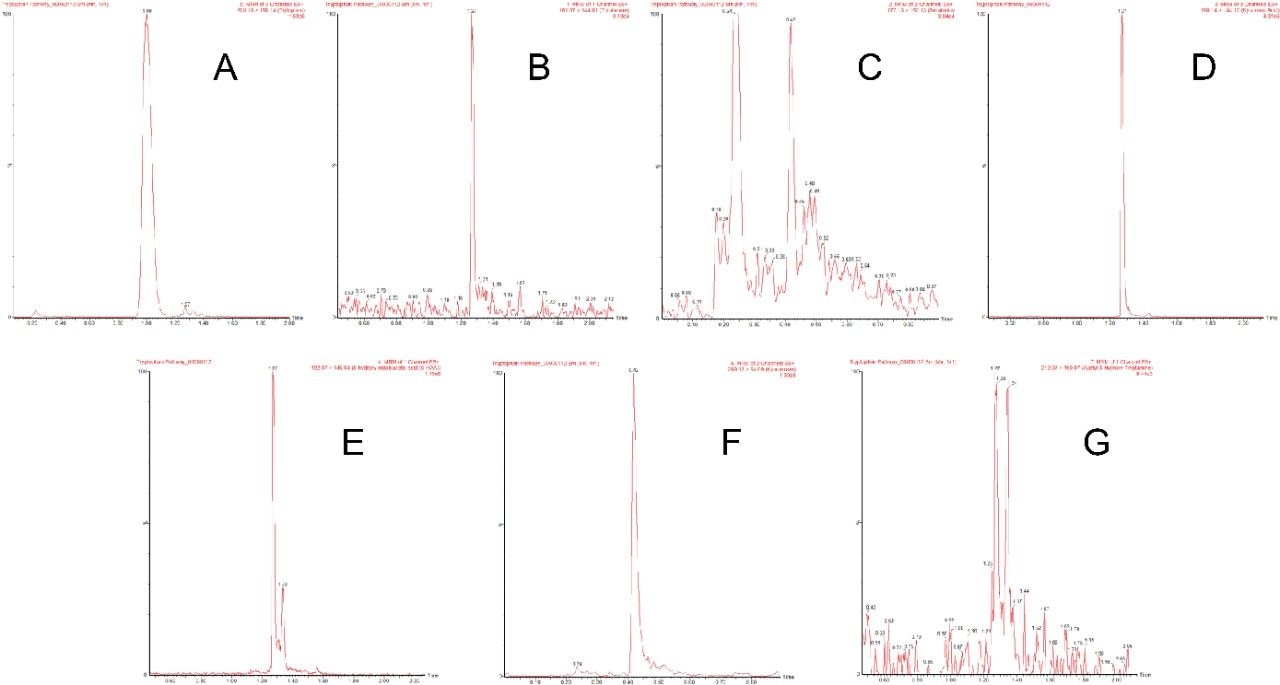
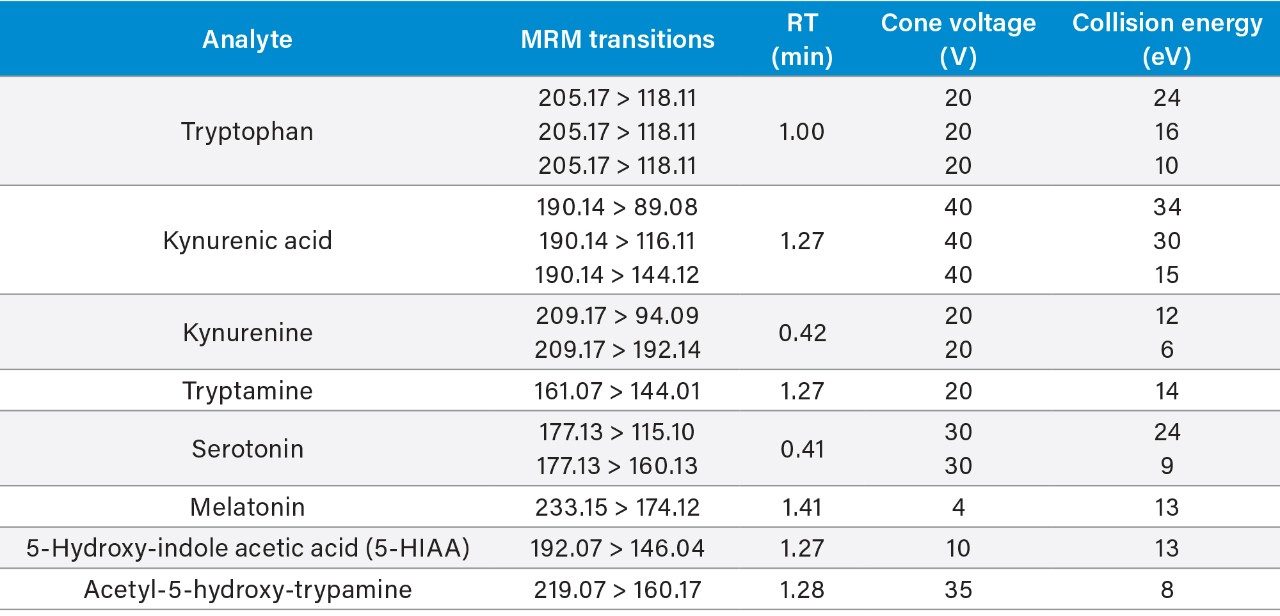
Tryptophan and its metabolites were separated and detected using the LC-MS platform and extraction protocols described above. Figure 2 shows an example chromatogram for the separation achieved using the method in a human urine sample. Peak identifications were confirmed using analytical standards. All analytes detailed in Table 1 were detected in human urine except for melatonin. Three analytes (serotonin, tryptophan, and kynurenine) were detected in human plasma. In Table 1, only one MRM transition is listed for some analytes whereas multiple MRM transitions are listed for other analytes. Both single and multiple MRMs gave similar levels of sensitivity. All the transitions were included for increased specificity.
A rapid UPLC-MS/MS methodology has been developed for the analysis of tryptophan and seven of its metabolites in various matrices. This research method has been demonstrated to be suitable for the analysis of physiologically relevant levels of these analytes in human plasma and urine. This method utilizes a generic, flexible LC-MS platform that can be used for various compound classes (including metabolomics, lipidomics, and proteomics), meaning it can be applied as part of a suite of analyses that are run sequentially as part of a targeted multi-omics workflow.
720006667, september 2019