For research use only. Not for use in diagnostic procedures.
A new analytical method is described for the analysis of TCA cycle metabolites as well as other related compounds without sample derivitization or ion-pairing reagents in the mobile phase. The method uses a simple mobile phase of water, acetonitrile, and 0.1% formic acid to achieve separation of 10 analytes in less than three minutes. The method was used to detect and identify analytes in human urine.
Non-ion pairing, mixed-mode method to analyze biologically relevant polar metabolites by UPLC-MS/MS.
The TCA cycle (also known as the Krebs cycle or Citric acid cycle) is the ultimate fate of metabolism where Acetyl-CoA, or other intermediates formed by the breakdown of carbohydrates, protein, and fat are enzymatically oxidized to produce energy and reduce cofactors to make ready for use in other enzymatic processes. Further, the TCA cycle produces precursors for amino acid, protein, fatty acid, cholesterol, and nucleotide synthesis for cell growth and division and represents a key component of homeostasis.¹ Regulation of the TCA cycle is largely based on product or substrate concentration as well as other inhibitors. Research into these analytes can provide insights into mechanisms which lead to diseases and disorders. Current separations include HILIC,² ion-pairing,³ anion exchange,⁴ and derivitization followed by gas chromotography⁵ or liquid chromotography⁶ separation with each method presenting its own unique challenges. Here we present a new research method for the analysis of the TCA cycle metabolites as well as other related compounds without sample derivitization or ion-pairing reagents in the mobile phase.
The components of the TCA cycle are small and very polar organic carboxylic acids. Traditional methods of reversed phase chromatography do not always yield enough retention or selectivity to confidently measure these analytes. Citric acid and isocitric acid, for example, are isobaric at 191 m/z and although malic acid has an m/z of 133, in-source fragmentation followed by decomposition in the collision cell gives rise to the same MRM transition as fumaric acid.³ To address the separation and retention of the critical pairs and very polar species of the TCA metabolites and other biologically relevant compounds, a mixed-mode chromatography method was developed. Here the ACQUITY UPLC CSH Phenyl-Hexyl 2.1 x 100 mm Column (p/n 186005407) was employed to separate lactic acid, malic acid, succinic acid, isocitric acid, citric acid, fumaric acid, pyruvic acid, α-ketoglutaric acid, phosphoenolpyruvic acid, and cis-aconitic acid, using a simple mobile phase of water and acetonitrile, each with 0.1% formic acid. Figure 1 shows the separation of the analytes at 80 °C with a flow rate of 0.4 mL/min and gradient of 2% B to 15% B over three minutes with detection performed using the Xevo TQ-S micro tandem mass spectrometer in negative ionization mode. Figure 2 shows the separation of the critical pairs: citric acid and isocitric acid, as well as malic acid and fumaric acid. Method reproducibility over different column lots was determined using a Method Validation Kit (p/n 186005580). Retention time reproducibility was less than 1.3% RSD for all of the analytes tested (Table 1). Finally, the method was tested on human urine samples by dilution with water 10 x. A sample of 3 µL was injected onto the column with the TargetLynx XS results shown in Figure 3. Here we observe well resolved components of the TCA cycle and other polar metabolites with acceptable peak shape in under three minutes.
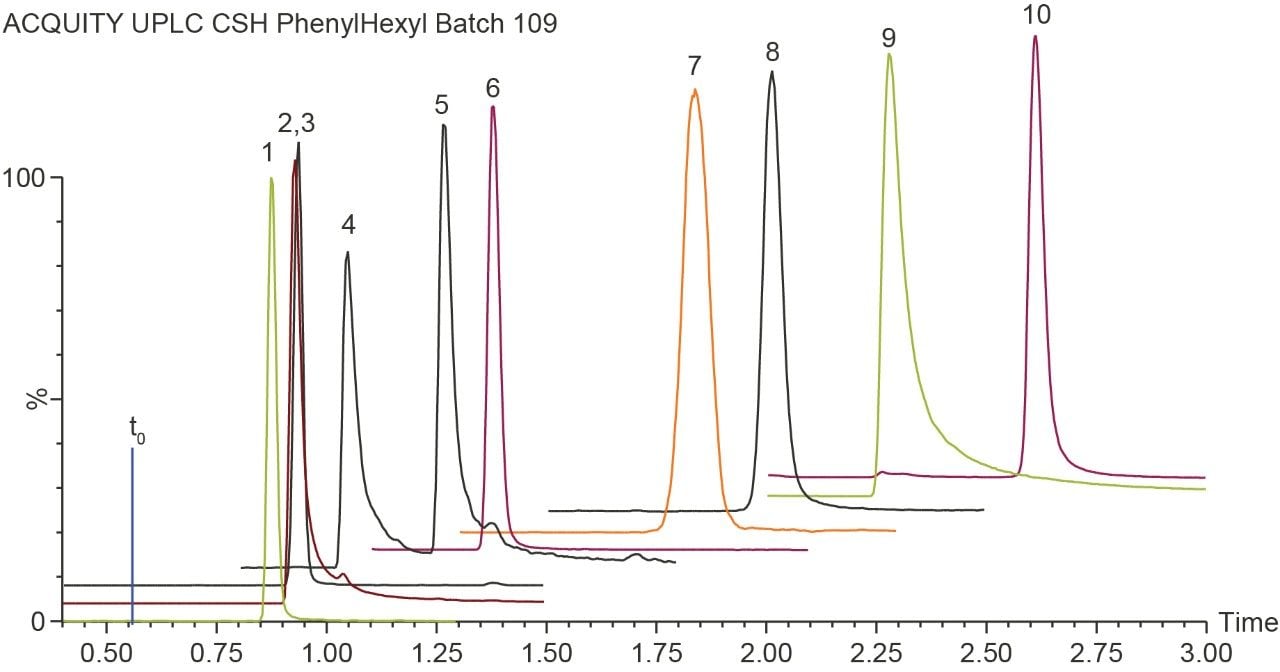
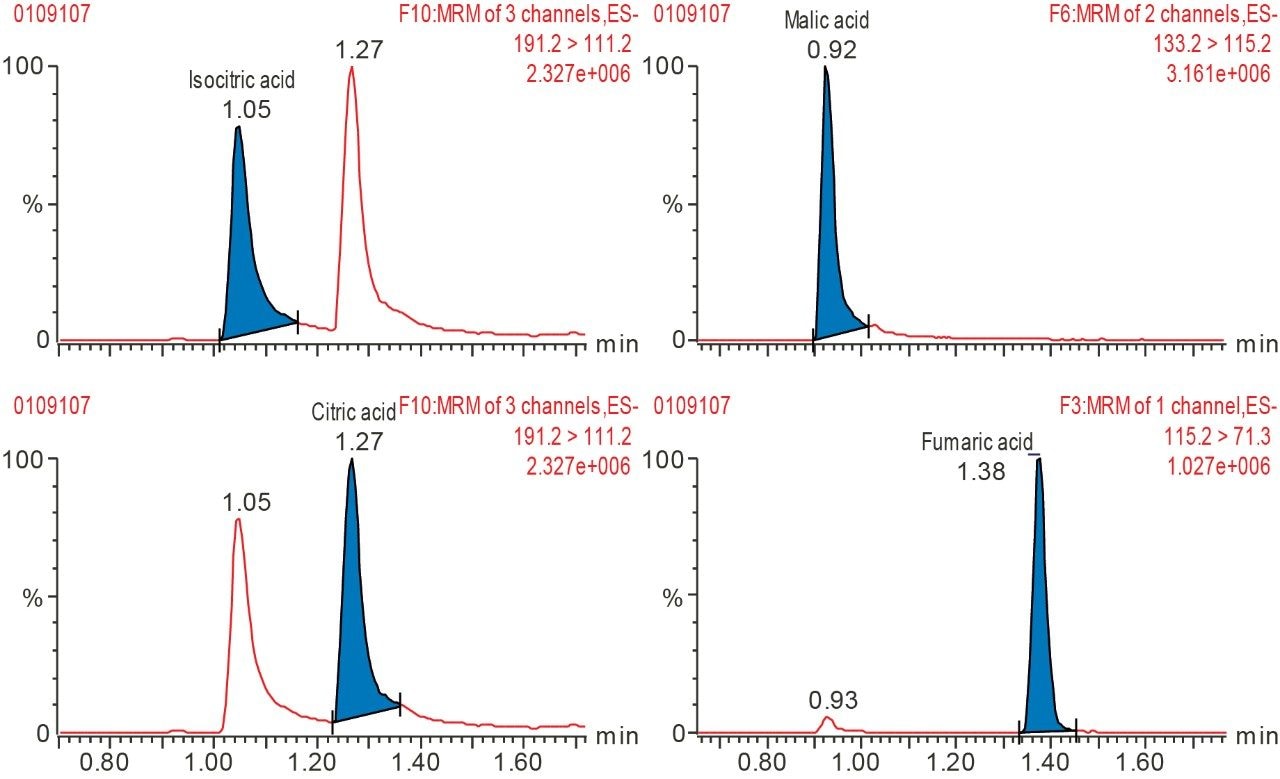
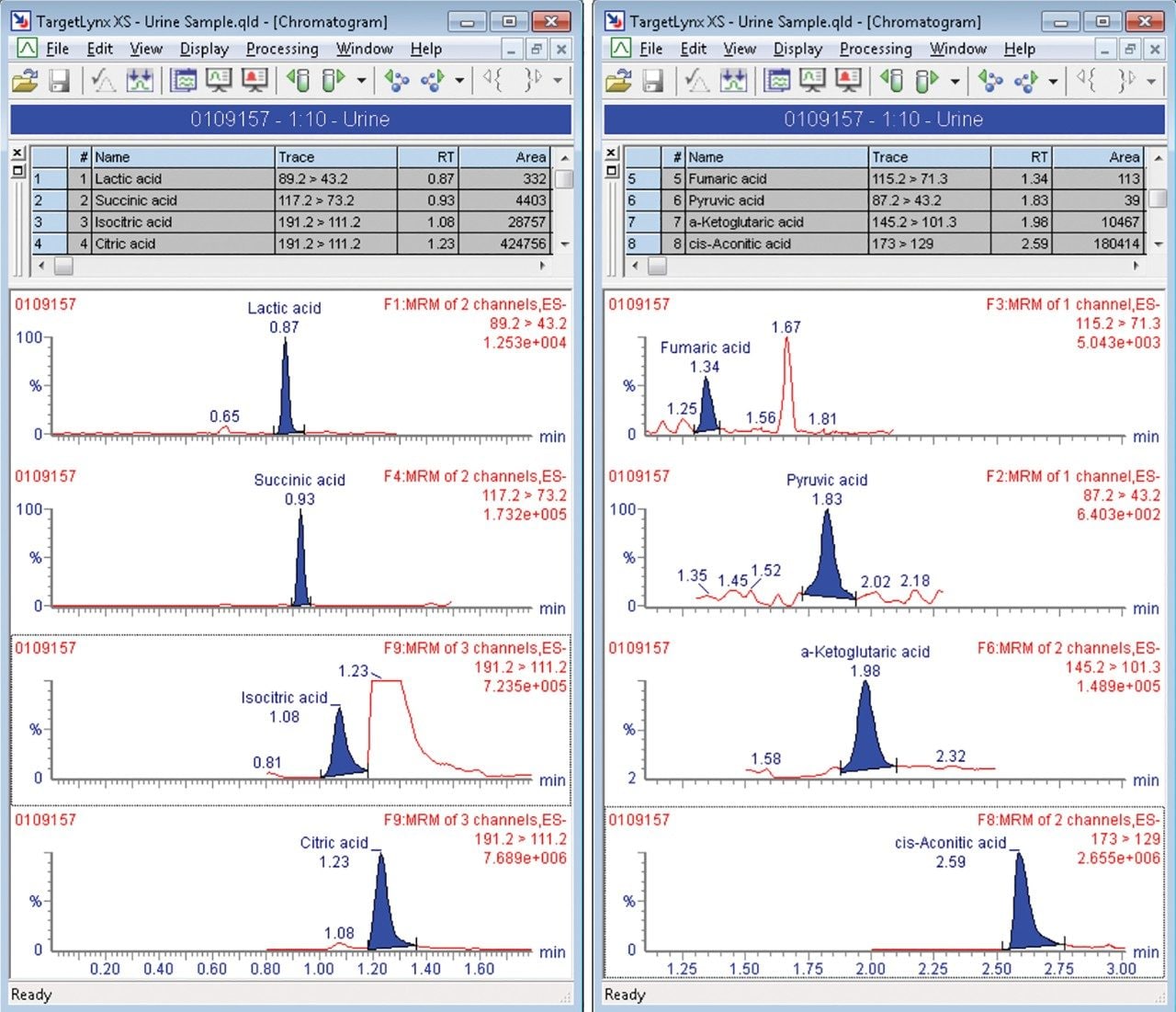
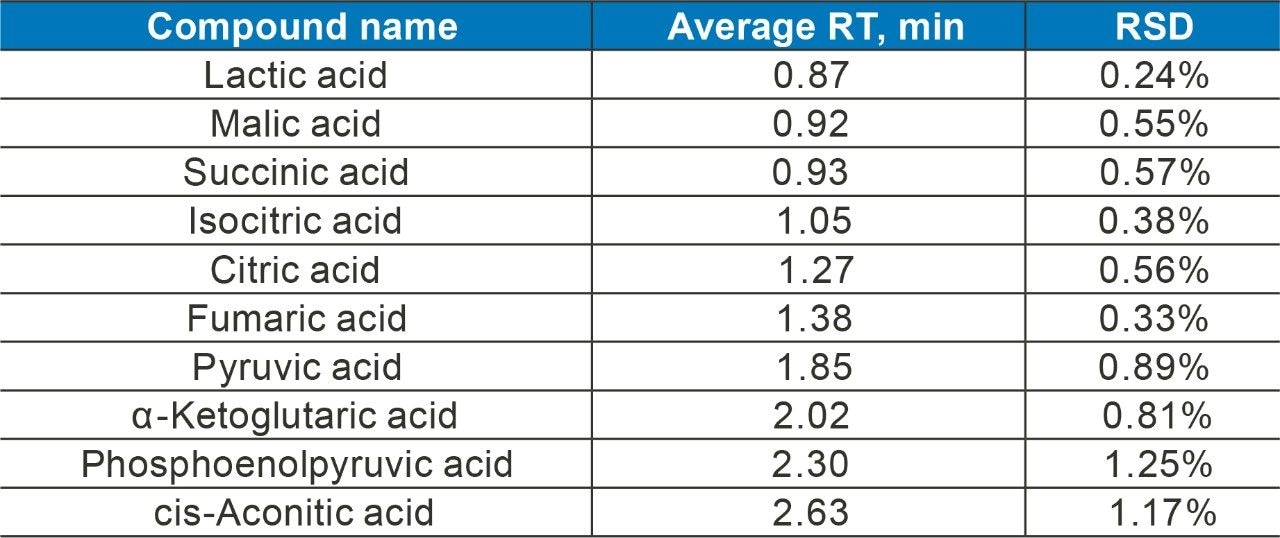
A research method for the separation and detection of TCA cycle metabolites as well as other related organic acids has been developed. The method uses a simple mobile phase of water, acetonitrile, and 0.1% formic acid to achieve separation of 10 analytes in less than three minutes. The method was used to detect and identify analytes in human urine for clinical research.
720006463, February 2019