This is an Application Brief and does not contain a detailed Experimental section.
The COVID-19 pandemic has resulted in the mobilization of scientific resources and expertise globally. Waters COVID-19 Innovation Response Team was formed to collaborate with partner organizations on analytical efforts supporting the fight against the novel coronavirus. As a result of these collaborations, Waters created a resource hub www.waters.com/c19 to collate and share technical information that may be useful for analytical scientists in their efforts against COVID-19. In this brief, we provide some high-level context to explain the use of different analytical technologies for COVID-19 related efforts.
An understanding of the use of LC and LC-MS technologies mobilized in the fight against COVID-19
Organizations across the globe have risen to the challenge of fighting the COVID-19 pandemic. To serve these organizations, Waters formed a COVID-19 Innovation Response Team intended to assist with our partners’ analytical challenges. To date, Waters has collaborated on 38 different projects. Based on the challenges and solutions that have emerged from these collaborations, Waters has compiled numerous technical resources which we believe can aid others in their fight against the novel coronavirus. These resources are available on www.waters.com/c19 and will be updated periodically. To date, Waters analytical support spans five critical applications areas:
Here, we briefly discuss the role of LC and/or LC-MS for each of these applications along with a sampling of select application notes. Our hope is that by providing a COVID-19 resource hub, organizations can quickly access relevant knowledge useful for their analytical pursuits against the novel coronavirus.
Protein-based vaccines have emerged as a promising approach to combat COVID-19. With this modality, a recombinant protein antigen is expressed, purified, and formulated to be dosed as a vaccine, often with an adjuvant to solicit an immunological response in patients.
Analytical characterization by LC-MS is essential to understanding vaccine properties, functions, and facilitating fast, reliable decision-making during all phases of vaccine development processes. More specifically it enables accurate, robust monitoring and control of key quality attributes of protein vaccines (e.g., structure, content, and identity), ensures robust construct development, and guides efficient biomanufacturing, purification, and formulation development.
Furthermore, identification of the target candidates that bind with a protein antigen (vaccine) relies on investigations into fundamental SARS-CoV-2 structural biology. One promising target is the SARS-CoV-2 spike protein. As with mAbs, LC, and LC-MS based techniques are useful to understand N-glycosylation profile, perform peptide mapping, intact mass analysis, and identify the antigen epitope.
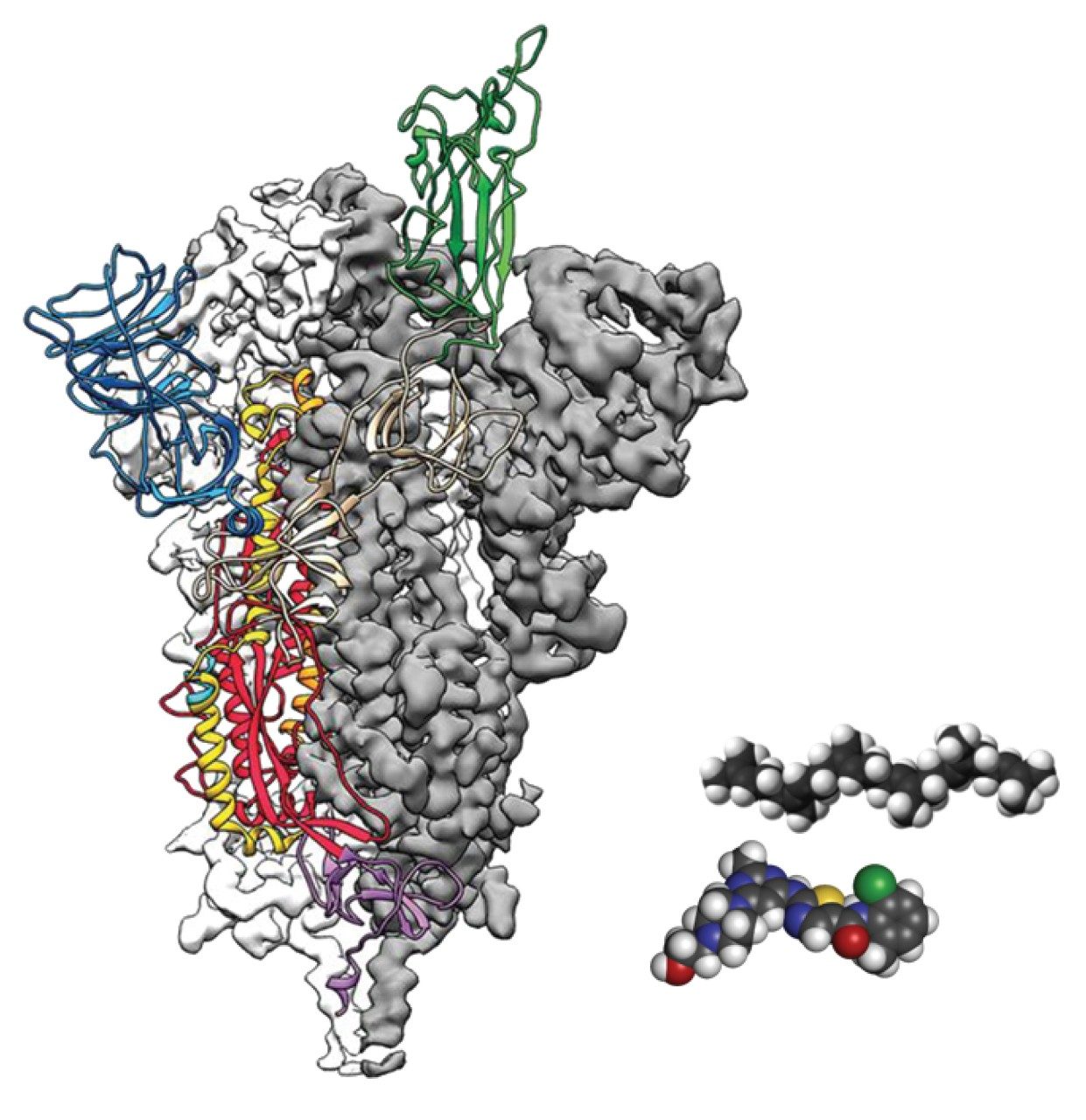
For further reference, please see Waters Application Notes mentioned below.
Comprehending COVID-19: Rapid and Sensitive Characterization of N-Glycans from SARS-CoV-2 Spike Protein, 720006914EN.
Comprehending COVID-19: Preliminary Examination of the SARS-CoV-2 Spike Protein by Peptide Mapping, 720006909EN.
Comprehending COVID-19: Reversed-Phase Liquid Chromatography (RPLC) of Intact SARS-CoV-2 Spike Protein, 720006907EN.
Enhanced Performance of the SYNAPT XS and Its Impact on Hydrogen Deuterium Exchange Mass Spectrometry (HDX MS) Data Quality, 720006870EN.
Timely diagnosis of SARS-CoV-2 viral infection remains paramount to successfully managing the COVID-19 pandemic. PCR-based approaches are facilitating qualitative or quantitative detection of SARS-CoV-2 viral genetic code in countless laboratories around the world. These diagnostics rely on high quality and high purity oligonucleotides that serve as primers and probes during amplification and detection.
Quality control via LC or LC-MS technologies is important to ensure the identity and purity of oligonucleotide probes and primers to meet the required quality standards to ultimately produce accurate diagnostic results.

As an established therapeutic modality, monoclonal antibodies (mAbs) are now being mobilized in the fight against COVID-19. Like all biotherapeutics, detailed analytical characterization of critical quality attributes is essential to guide their development. Neutralizing antibodies are being developed to provide options for prophylaxis and to confer passive immunity. In addition, antibodies that can dampen effects from cytokine storms are expected to play an important role in the treatment of COVID-19.
LC or LC-MS based technologies are among the most effective tools to characterize these COVID-related mAbs including higher-order structure analysis, size and charge variant analysis, peptide mapping, N-glycan analysis, and intact mass analysis (native and denatured). It is possible that some alternative versions of techniques can also be applied, including subunit profiling and native LC-MS approaches based on SEC-MS and IEX-MS.

For further reference, please see Waters Application Note: Online IEX-MS of mAb Charge Variants Using a BioResolve SCX mAb Column, IonHance CX-MS pH Concentrates, and BioAccord System, 720006672EN.
Nucleic acids are being used to harness a recipient’s own body to transcribe and translate antigenic protein components from viral genetic sequence. mRNA and vectorized DNA are the newest types of vaccines being pursued to fight against COVID-19 because of their potential fast scale up readiness. Whether it be a nanoparticle encapsulated mRNA or vectorized DNA, these new modalities need to be characterized and QC tested to ensure safety and efficacy.
Modern, powerful analytical techniques such as LC-MS are currently being applied in the characterization of the mRNA/DNA based vaccine candidates, components, and intermediates. Viral mRNA is often synthetically produced. These large molecules can be digested by endonucleases and the fragments can be analyzed by LC-MS. LC-MS analysis provides high-confidence mRNA sequence identification and verification. Further, LC-MS can detect and quantify the low-level sequence impurities such as SNPs. An additional example using an intact mass confirmation workflow is the analysis of the capped to uncapped ratio of mRNA fragments, which is critical for stability/translation.

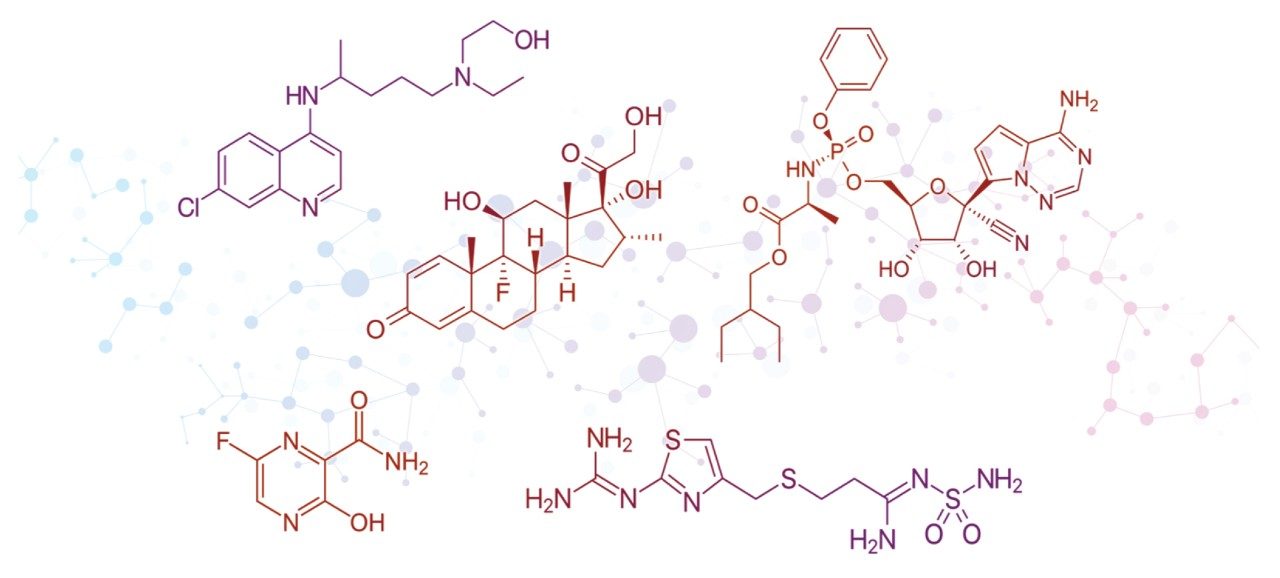
Many small molecules are being or were examined for their ability to inhibit the replication and transmission of SARS-CoV-2. These molecules range from repurposed anti-malarial drugs and over-the-counter heartburn medication to polymerase and protease inhibitors.
LC and LC-MS tools are utilized during release testing, formulation studies, clinical stage bioanalysis, and therapeutic drug monitoring. Each of these applications is critically important to readying these active pharmaceutical ingredients, demonstrating their effectiveness, and determining the specialized dosing schedules to patients in distress.
LC and LC-MS are valuable analytical tools supporting the development of various therapeutic modalities and diagnostics in the fight against COVID-19. As a result of numerous collaborations, Waters provides this brief summary of the critical uses of LC and LC-MS technology. In combination with this brief overview, Waters hopes that specific technical resources available on www.waters.com/c19 will enable analytical scientists to quickly access the information needed in their efforts against the novel coronavirus.
720007041, Revised November 2020