Aflatoxins and ochratoxin A can occur naturally in a variety of food products via pre- and post-harvest contamination mechanisms and are regulated worldwide. Consumption of mycotoxin contaminated foods can pose a significant risk to human health. Herein we describe three different methods for specific food commodities:
All the mentioned methods have been internally validated using spiked samples as well as naturally contaminated reference materials, where available, and are based upon a solid-liquid extraction, followed by a trap-and-release step using immunoaffinity chromatography (IAC) and LC-MS/MS analysis.
The methods provide optimal performance in terms of trueness and repeatability, with method LOQs as low as 0.050 µg/kg for aflatoxins and 0.4 µg/kg for ochratoxin A.
The use of IAC clean-up is particularly advantageous as it allows the use of calibration curves based on solvent standards, removing the need to prepare closely matrix-matched calibration standards and without the need for (isotopically labeled) internal standards. Moreover, it provides excellent recoveries (in the range 71–108%, mean 90%) due to the highly specific antibody-based binding, and minimizes the potential matrix effects, thus making it suitable to be coupled with tandem mass spectrometry.
The determination of aflatoxins and ochratoxin A in agricultural crops, for food and feed, is a priority for both the industry and competent authorities alike to check compliance with strict regulatory limits. In addition to the international Codex standards,1 many countries have their own national mycotoxin legislation due to the risk consumption of contaminated foods poses to human health. The European Union has some of the most stringent Maximum Permitted Limits in the world and is advised on emerging risks by the EFSA panel on Contaminants in the Food Chain.2,3 The use of the immunoaffinity chromatography (IAC), based on antibodies, has become a popular approach for mycotoxin analysis. Immunoaffinity columns are pre-packed with a polymeric gel phase to which antibodies (having characterised cross reactivity against specific target compounds) are covalently immobilized. When the extract passes through the column, the target mycotoxin binds selectively to the antibodies, while other co-extractive components will be removed by a washing step.4 The mycotoxin is then eluted with a miscible solvent such as methanol which causes antibody denaturation. Due to their high specificity, immunoaffinity columns produce cleaner extracts when compared to less selective SPE sorbent materials and so are less susceptible to interference from co-extractives. The combination of clean-up using IAC and analysis by HPLC with fluorescence detection has been successfully used as a cost-effective way to check compliance with the regulatory limits for aflatoxins for many years.5,6 However, IAC columns can also be used with LC-MS/MS to provide added sensitivity, for example for the very low regulated levels for foods intended for infants and young children, to cope with analysis of the more complex commodities, and to simplify quantification by avoiding matrix-matched calibration or reliance on internal standards.7 Here we show performance of three methods: aflatoxins in various types of nuts, ochratoxin A in coffee and cocoa, and aflatoxins and ochratoxin A in black pepper.
Peanuts and pistachios were purchased at a local market. About 300 g of each food commodity were ground and homogenized using an analytical mill (IKA) prior to extraction. Hazelnuts reference materials were provided by Fera Science Ltd (FAPAS T04390QC) in the form of a homogenized water/nuts slurry. The assigned values and range of concentrations of aflatoxins in the FAPAS QC material are listed in Appendix A.
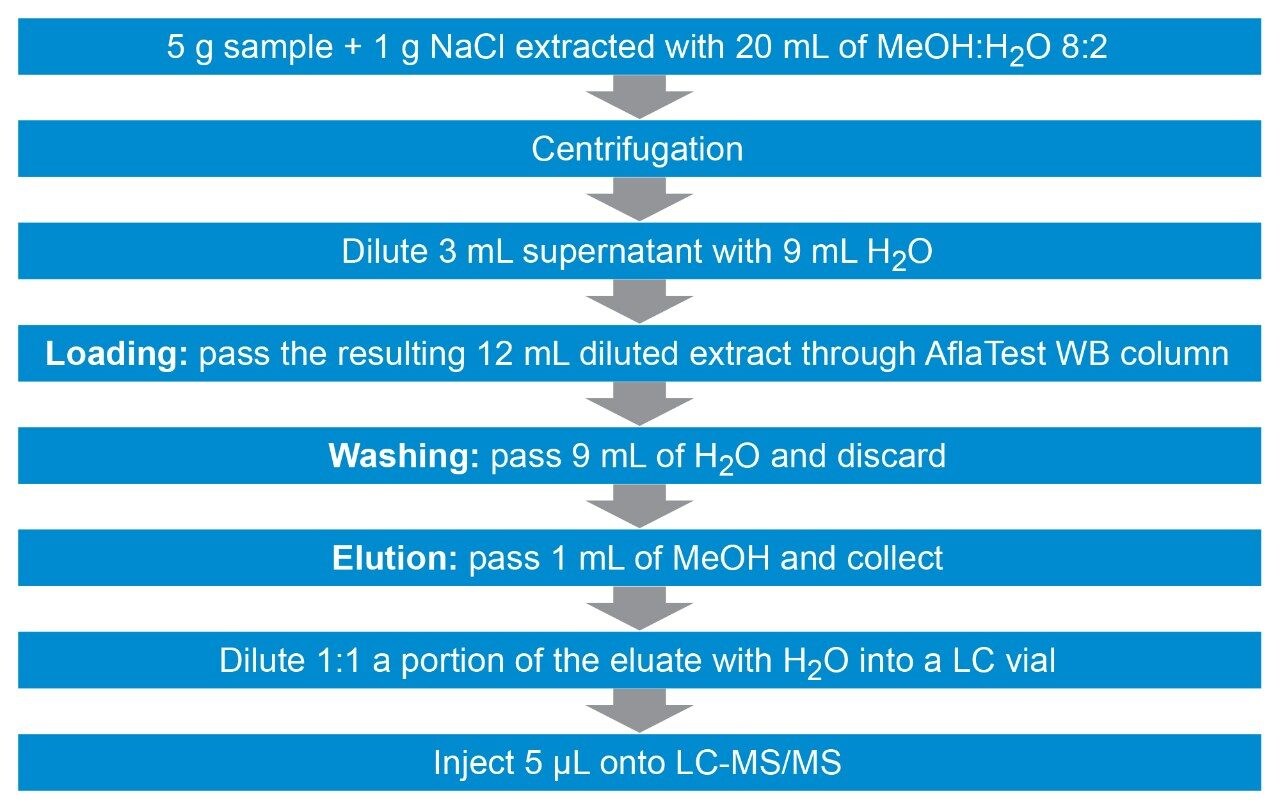
Before extraction, 5.0 ±0.01 g of homogenized solid sample and 1 g of sodium chloride are placed in a 50 mL falcon tube. 20 mL of MeOH:H2O 8:2 (v/v) are added and the tube is vigorously shaken for 10 seconds and placed in an automatic Vortex mixer for 10 minutes. After centrifugation at 5000 rpm (~5300 g) for 6 minutes, 3 mL of supernatant are diluted with 9 mL of water. Twelve mL of diluted extract are passed through VICAM AflaTest WB column (p/n G1024) at a rate of 1 drop/second (loading step). Then, 9 mL of water are passed through the column at a rate of 1–2 drops/second (washing step) and about 3 mL of air are pumped through. Aflatoxins are eluted by passing 1 mL of MeOH through the column at 1 drop/second or less. Finally, 500 µL of the eluent are mixed with 500 µL of water in an LC vial prior to LC-MS/MS analysis. The resulting dilution factor is 2.67 (8/3).
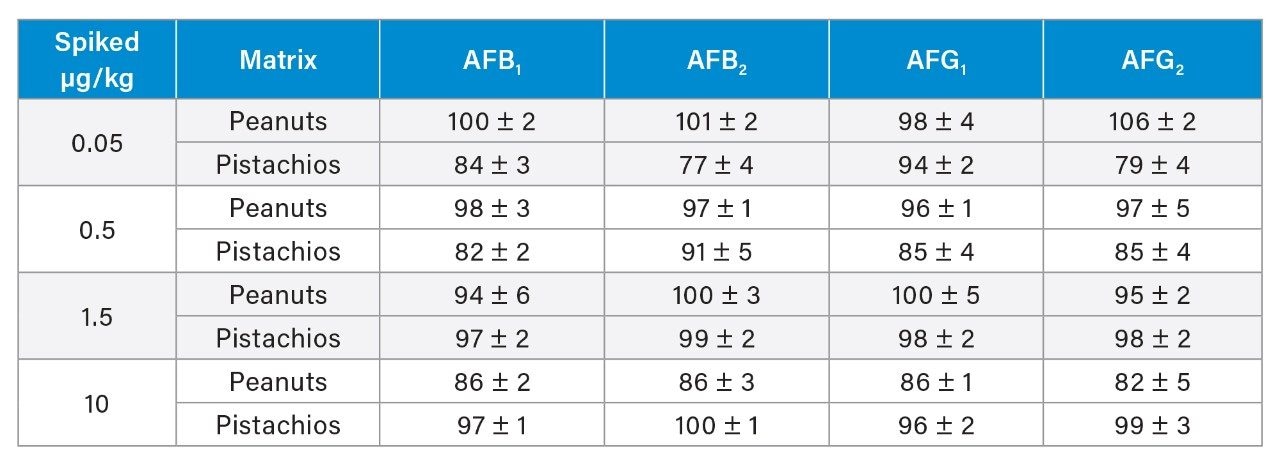
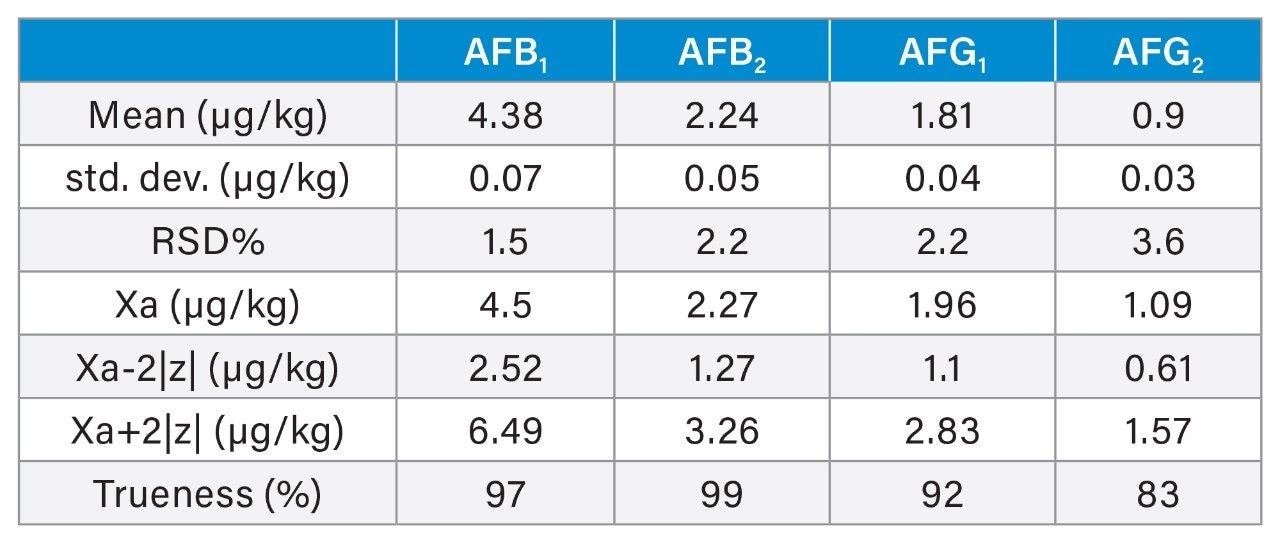
A recovery experiment was performed by spiking peanuts and pistachios samples with a mix of aflatoxins at four concentration levels (0.05, 0.5, 1.5, and 10 µg/kg of each analyte) in triplicate. Blanks and spiked samples were then extracted and analyzed as described in the previous section. Trueness and precision were also verified by analyzing hazelnut reference materials in six independent replicates.
Roasted coffee beans and cocoa powder were purchased at a local market. About 300 g of coffee beans were ground and homogenized using an analytical mill (IKA) prior to extraction. A bulk sample of cocoa powder was prepared by mixing portions of different samples. Coffee and cocoa were screened using LC-MS/MS prior to method validation and no detectable levels of OTA were reported.
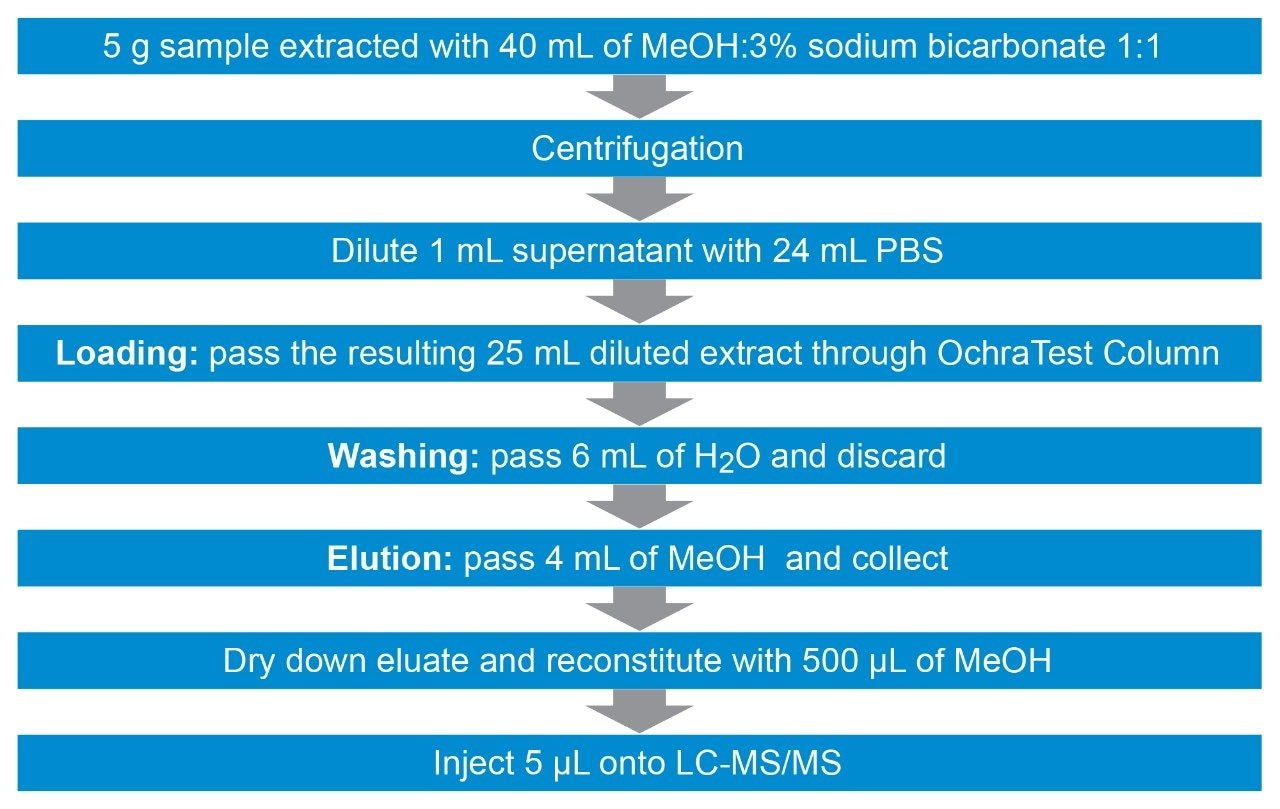
5.0 ±0.01 g of homogenized solid samples are weighed in a 50 mL plastic centrifuge tube. Forty mL of the extraction solution consisting of a 50:50 (v/v) mixture of MeOH:H2O with 3% (w/v) of sodium bicarbonatea are added. The tube is placed in an automatic vortex for 10 minutes. After centrifugation at 5000 rpm (~5300 g) for 10 minutes, the supernatant is diluted 1:25 by mixing 1 mL of extract with 24 mL of phosphate buffer solution (PBS)b in a 30 mL glass vial, or equivalent. The diluted extract (25 mL) is passed through VICAM OchraTest WB Column (p/n G1033) at a rate of 1–2 drops/second (loading step). Then, 6 mL of water are passed through the column at the same rate (washing step) followed by pumping air through (about 3 mL). Ochratoxin A is eluted by passing 4 mL of methanol through the column at 1 drop/second or less and collecting. The eluate is dried down under nitrogen at 40 ºC. The residue is reconstituted with 500 µL of MeOH:H2O 50:50 (v/v) and transferred into a LC vial prior to LC-MS/MS analysis. The resulting dilution factor is 4.
(a) Preparation of the extraction solution: To a 1 L glass bottle add 15 g of sodium bicarbonate and 500 mL of purified water. Mix well and place the bottle into an ultrasound bath until complete dissolution of the salt. Add 500 mL of methanol and mix well.
(b) Preparation of PBS: To a 1 L glass bottle add 100 mL of VICAM 10x concentrate PBS (p/n G1113) and 900 mL of purified water (pH 7.0).
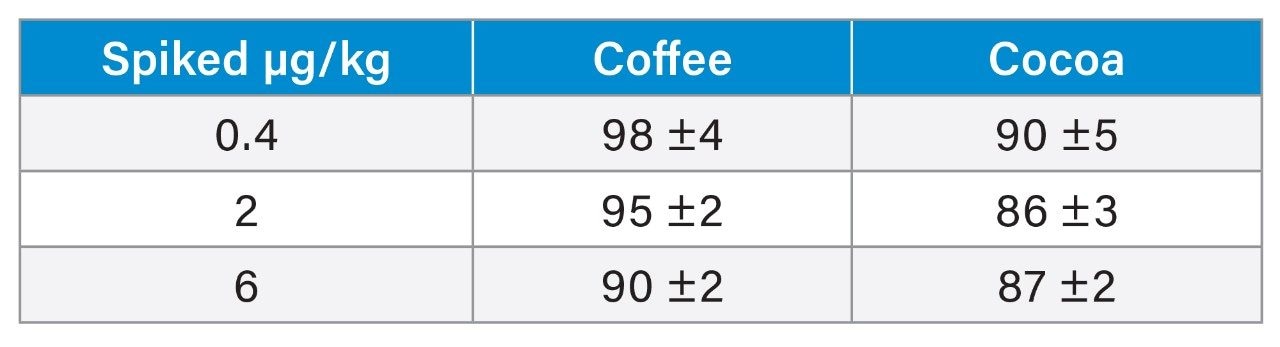
Trueness was verified by a recovery experiment where powdered coffee and cocoa samples were spiked with ochratoxin A at three concentration levels (0.40, 2.0, and 6.0 µg/kg) in triplicate. Blanks and fortified samples were then extracted and analyzed as described in the previous section. Six different blank coffee and cocoa samples were fortified at 6.0 µg/kg of OTA and RSDr% were calculated as an intra-day repeatability parameter.
Black peppercorns were purchased at a local market. About 200 g of sample were ground and homogenized using an analytical mill (IKA) prior to extraction. Powdered black pepper reference materials were provided by Fera Science Ltd. (FAPAS T04332QC). The assigned values and range of concentrations of aflatoxins and ochratoxin A in the FAPAS QC material are listed in Appendix A.
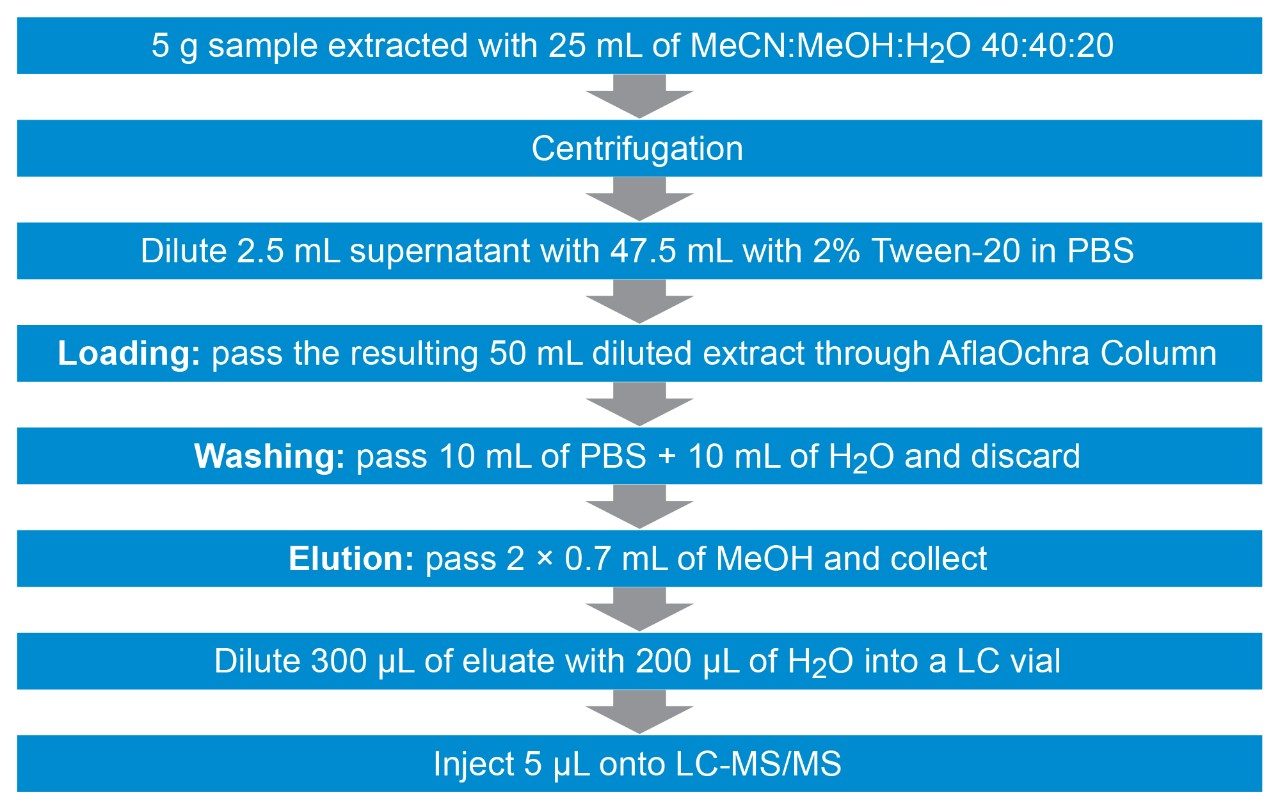
Before extraction, 5.0 ± 0.01 g of homogenized solid samples are placed in a 50-mL falcon tube. Ten mL of MeCN:H2O 84:16 (v/v) are added and the tube is vortexed for 3 minutes. Fifteen mL of MeOH:H2O 80:20 (v/v) are added and the tube is vortexed for an additional 3 minutes. After centrifugation at 5000 rpm (~5300 g) for 6 minutes, 2.5 mL of supernatant are diluted with 47.5 mL of 2% (w/w) Tween-20 in phosphate buffer solution (PBS)a. The entire diluted extract (50 mL) is passed through a VICAM AflaOchra Column (p/n G1017) at a rate of 1 drop/second (loading step). Then, 10 mL of PBSb are passed through the column at a rate of 1-2 drops/second (washing step 1) and an additional 10 mL of water are passed through (washing step 2). About 3 mL of air are slowly pumped to dry the cartridge. 0.7 mL of MeOH are passed through at 1 drop/second and collected (elution 1). The column is let to run dry without pumping extra air through and let to stand for 1 minute. An additional 0.7 mL of MeOH is passed through (elution 2) and collected with the previous portion by forcing 10 mL of air through. Finally, 300 µL of eluate extract are mixed with 200 µL of water directly into an LC vial prior to LC-MS/MS analysis. The resulting dilution factor is 4.67 (14/3).
(a) Preparation of the diluent (2% Tween-20 in PBS): weigh 20 g of Tween-20 in a 1 L glass bottle. Add 100 mL of VICAM 10X concentrate PBS (p/n G1113) and 900 mL of purified water. Mix well and place the bottle into an ultrasound bath for 10 minutes.
(b) Preparation of PBS: To a 1-L glass bottle add 100 mL of VICAM 10x concentrate PBS (p/n G1113) and 900 mL of purified water (pH 7.0).
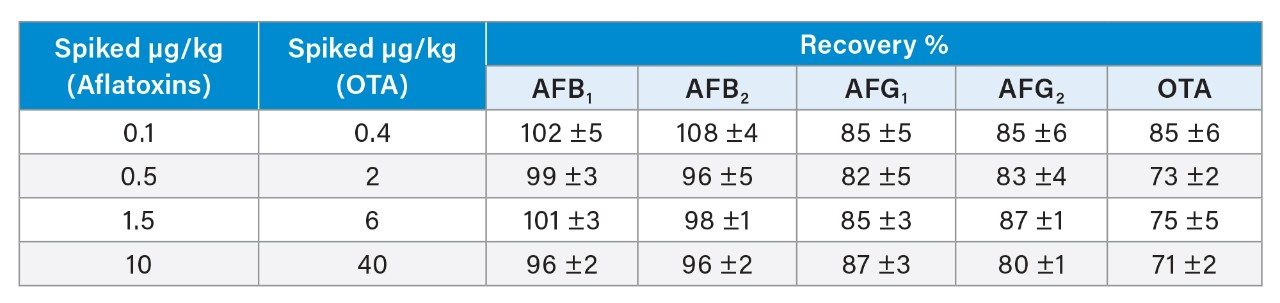
A recovery experiment was performed by spiking separated black pepper samples with a mix of aflatoxins and OTA at four concentration levels (0.1, 0.5, 1.5, 10 µg/kg of each aflatoxin and 0.4, 2.0, 6.0, 40 µg/kg of OTA) in triplicate. Blanks and fortified samples were then extracted and analysed as described in the previous section. Trueness and precision were also verified by analysing a black pepper certified reference material in six independent replicates.
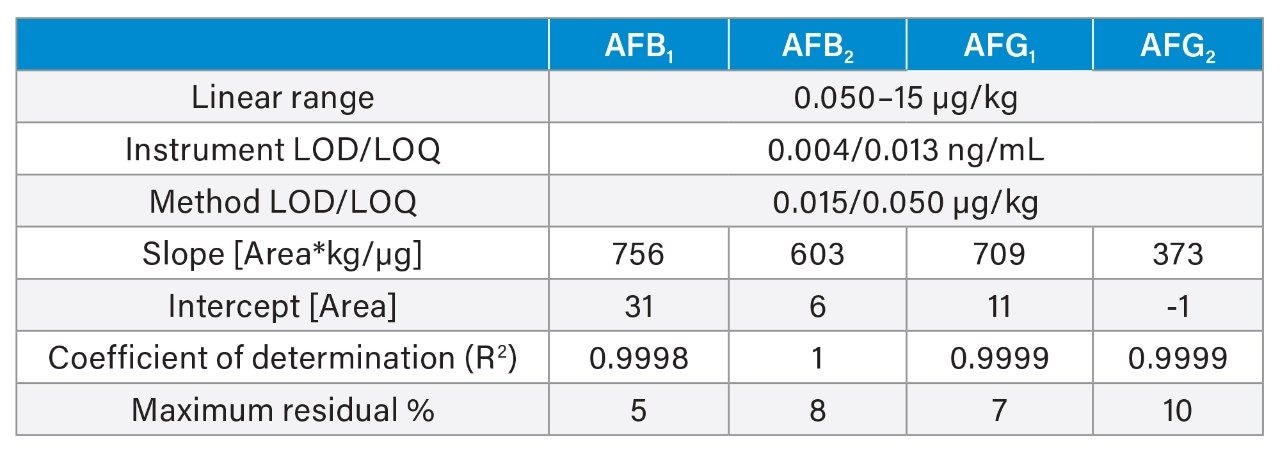
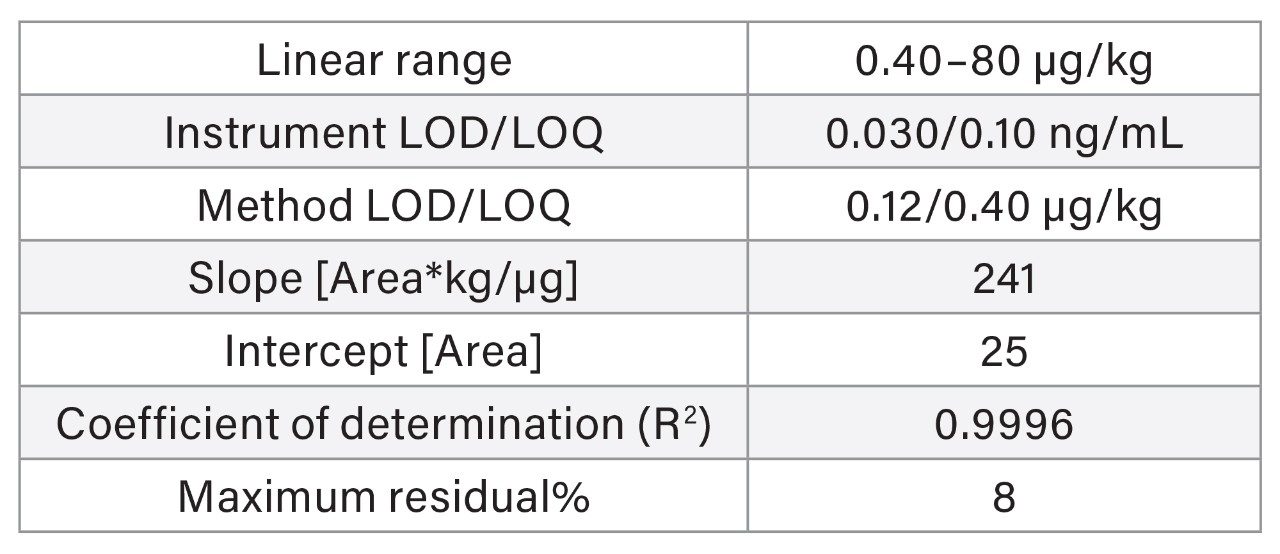
The linearity of the method was verified across the range of concentrations tested (0.050–13.3 µg/kg). Standard solutions were prepared in solvent (see Appendix A for preparation of standards). Table 1 reports the linearity parameters and the lower limit of detection (LOD) and quantitation (LOQ). The instrument LOQ was 0.013 ng/mL, while the method LOQ was 0.050 µg/kg for each aflatoxin. The EU maximum limits of AFB1 in peanuts, hazelnuts, and pistachios are 2.0, 5.0, and 8.0 µg/kg, respectively. Whereas the maximum limits for the sum AFB1+AFB2+AFG1+AFG2 are 4.0 in peanuts and 10.0 µg/kg in pistachios and hazelnuts.2 These limits lay within the linear range of the method.
Recoveries were between 82 and 106% in peanuts and between 77% and 100% in pistachios, across all levels (Table 2). Calculated concentrations in hazelnuts reference material were well between ±2|z|-score, with trueness between 83 and 99%. Precision was assessed under repeatability conditions, RSDr%, and ranged between 1.5 and 3.6% (n = 6), as shown in Table 3. Method performance in terms of recommended recovery for aflatoxins set by the EU Regulation are 50–120% for concentrations below 1.0 µg/kg, and 70–110% for concentrations within 1–10 µg/kg; while maximum RSDr% are set to be 14.5% (derived from the Horwitz equation).8–10 Method trueness and repeatability are therefore, in line with the European Regulation.
The linearity of the method was verified across the range of concentrations tested (0.40–80 µg/kg of solid sample). Standard solutions were prepared in solvent (see Appendix A for preparation of standards). Table 4 reports the linearity parameters and the lower limit of detection (LOD) and quantitation (LOQ). The instrument LOQ was 0.10 ng/mL, while the method LOQ was 0.40 µg/kg. The EU maximum limits of OTA in roasted coffee beans and ground roasted coffee is 5.0 µg/kg,2 which is well bracketed within the calibration range. Cocoa powder is currently under review by the EFSA Contam Panel and it is expected to be regulated in the future, where maximum limits, similar to coffee, are likely.
Recoveries ranged between 90 and 98% in coffee, and between 86 and 90% in cocoa (Table 5). Precision was assessed under repeatability conditions. The repeatability experiment (from the analysis of six independent replicates) leads to RSDr% of 1.0 and 2.1% for coffee and cocoa, respectively. The method performance in terms of recommended recovery for ochratoxin A set by the EU Regulation are 50–120% for concentrations below 1 µg/kg, and 70–110% for concentrations above 1 µg/kg; while maximum RSDr% are set to be 40% for concentrations below 1 µg/kg, and 20% for concentrations above 1 µg/kg.8 Method trueness and repeatability are therefore in agreement with the European Regulation.
The linearity of the method was verified across the range of concentrations tested (0.10–25 µg/kg for aflatoxins and 0.40–100 µg/kg for ochratoxin A. Standard solutions were prepared in solvent (see Appendix A for preparation of standards). Table 6 reports the linearity parameters and the lower limit of detection (LOD) and quantitation (LOQ). The instrument LOQ was 0.02 ng/mL for each aflatoxin and 0.08 ng/mL for ochratoxin A; while the method LOQ was 0.10 µg/kg for each aflatoxin and 0.40 µg/kg for OTA. The EU Maximum Permitted Limits for AFB1 and OTA in spices (including black pepper) are 5.0 and 15 µg/kg, respectively. Whereas the maximum limit for the sum AFB1+AFB2+AFG1+AFG2 is 10.0 µg/kg.2 These limits lay within the linear range of the method.
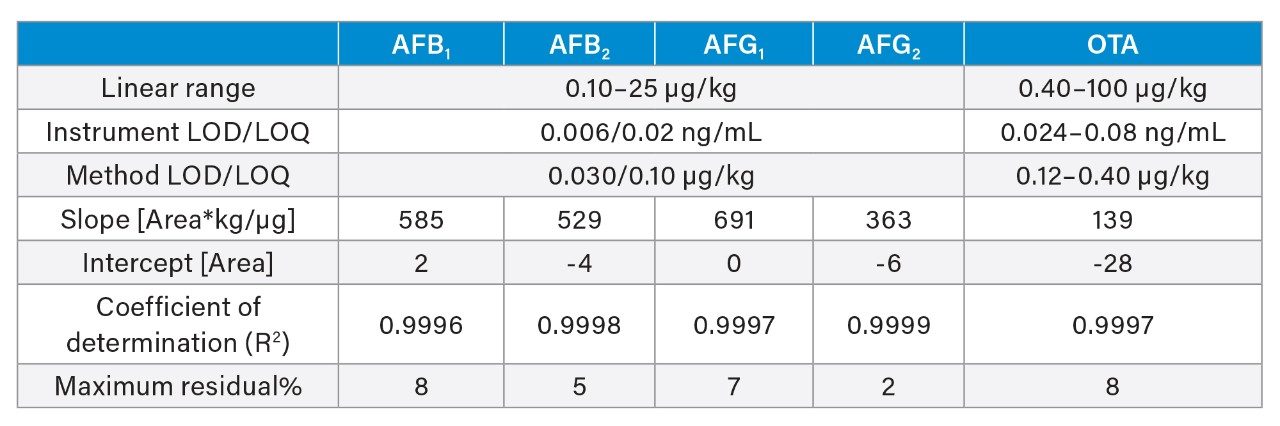
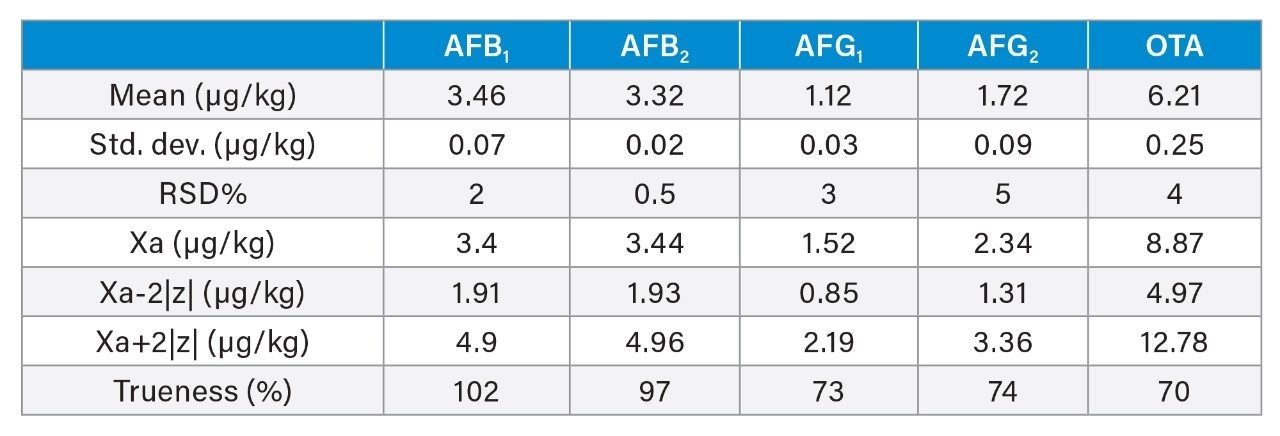
Recoveries were between 80 and 108% for each aflatoxin and between 71 and 85% for OTA, across all levels (Table 7). Calculated concentrations in black pepper reference material were between ±2|z| score, with trueness between 73 and 102% for each aflatoxin, and 70% for OTA. Precision was assessed under repeatability conditions, RSDr% ranged between 0.5 and 5% for each mycotoxin (n = 6), as shown in Table 8. Method performance in terms of recommended recovery for aflatoxins set by the EU Regulation are 50–120% for concentrations below 1.0 µg/kg, and 70–110% for concentrations within 1–10 µg/kg; while maximum RSDr% are set to be 14.5% (derived from the Horwitz equation).8-10 The method performance in terms of recommended recovery for ochratoxin A set by the EU Regulation are 50–120% for concentrations below 1 µg/kg, and 70–110% for concentrations above 1 µg/kg; while maximum RSDr% are set to be 40% for concentrations below 1 µg/kg, and 20% for concentrations above 1 µg/kg.8 Method trueness and repeatability are therefore, in accordance with the European Regulation.
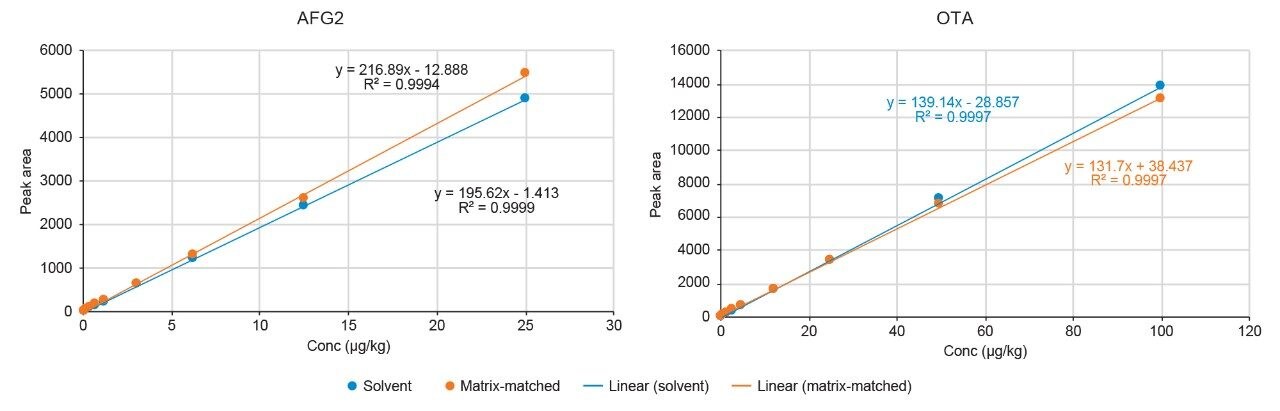
Matrix effect factor (MEF%) was assessed as the percentage ratio
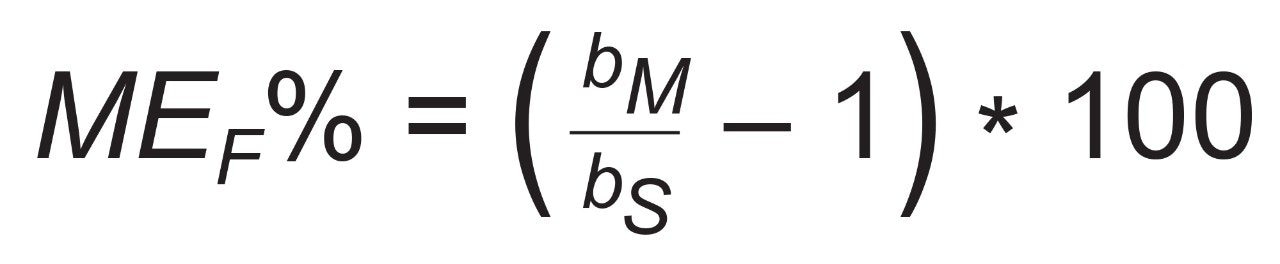
where bM and bS are the slope of the matrix-matched and solvent calibration curve, respectively. The matrix-matched calibration curve was obtained by plotting the response of the standards prepared by diluting the stock mycotoxins solution with blank matrix extract, following the same dilution procedure as the solvent standard preparation. Among the three described methods, Method 1 (aflatoxins in nuts) showed the lowest matrix effect (matrix effect factors ranging between -4% signal suppression to +2% signal enhancement). While method 3 (aflatoxins and ochratoxin A in black pepper) presented the highest matrix effects (matrix effect factors ranging between +11 and +13% signal enhancement for aflatoxins, and -5% signal suppression for ochratoxin A). It is important to note the drastic reduction of matrix effect compared to a previously reported dilute-shoot method, which presented MEF% >100% signal enhancement for ochratoxin A in cereals.11 As an example, in Figure 4 are reported the calibration plots of aflatoxin G2 and ochratoxin A in black pepper. It is apparent that the herein encountered matrix effects are not significantly impacting the quantitation results for the tested analytes. The reduction of matrix effects by the use of immunoaffinity chromatography is extremely beneficial as it allows the determination of method recoveries without the need to compensate for matrix effects or prepare matrix-matched calibrants as accurate results are achieved by using solvent standards.
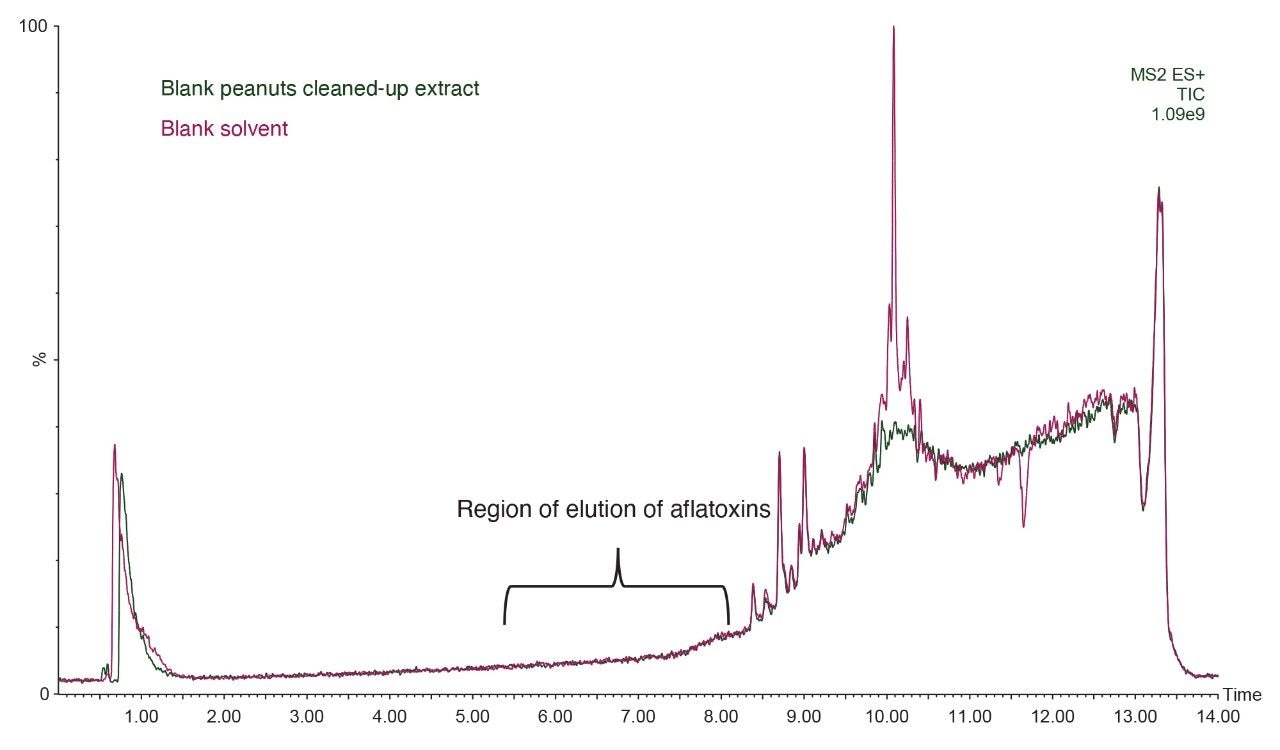
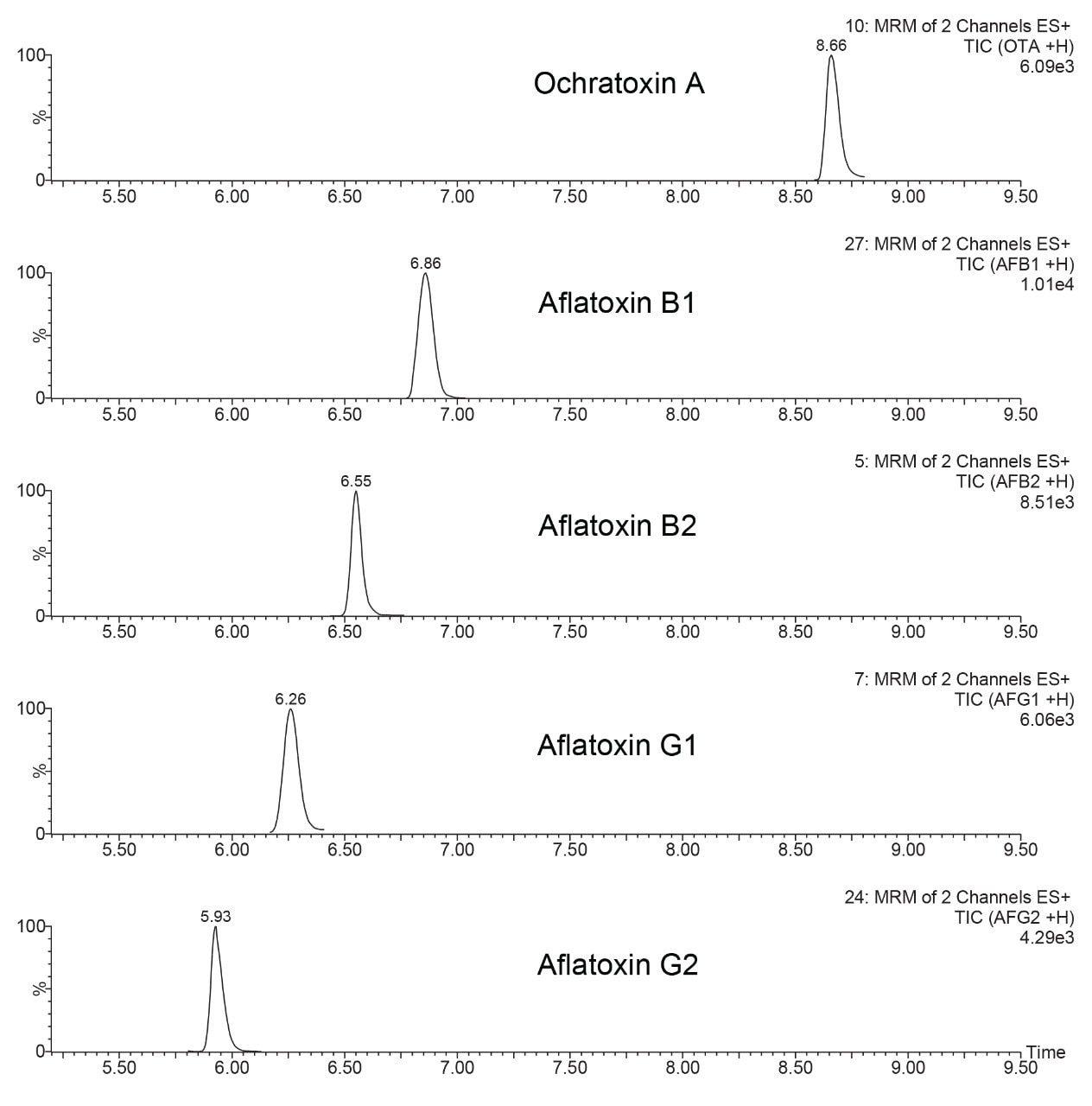
In Figure 5 is shown ESI+ SCAN chromatograms of the solvent blank and peanuts extract after IAC clean-up, highlighting the elution region of aflatoxins. It is evident that there are no additional co-extractives present in the chromatogram interfering with the analysis or competing for charge in electrospray ionization source region. This provides further evidence of the effective removal of matrix components when using the immunoaffinity chromatography.
The performance of the three methods described fulfil the criteria set by the European Regulation and SANTE guidelines.12 The main advantage of the use of immunoaffinity chromatography clean-up is that it is possible to use calibration standards prepared in solvent, without the use of closely matrix matches standards, and it avoids labeled internal standards for reliable quantitation. This is important as it is usually difficult to obtain a true blank due to the typical hot-spot contamination. The methods presented very good repeatability, and trueness have been verified via recovery experiments as well as using incurred reference materials with known concentrations of mycotoxins. Matrix effects were also evaluated, and it has been found that potential matrix-induced signal suppression/enhancement are minimal (<|13|%) and have negligible impact on the quantitative performance.
Finally, the methods could be extended to wider types of food commodities, similar to those tested in this work.
We thank Nancy Zabe Collette, Danrey Toth, and Chiara Bottesini for their assistance with this study.
The LC-MS/MS conditions were based on the previously described multi-mycotoxins method (App Note p/n 720006685en).11
|
System: |
ACQUITY UPLC I-Class (BSM) with FTN autosampler |
|
Column: |
ACQUITY UPLC BEH C18 (2.1 × 100 mm, 1.7 µm particle size, 130 Å pore size, p/n 186002352) |
|
Aqueous mobile phase: |
1 mM ammonium acetate in water +0.5% acetic acid +0.1% formic acid (v/v) |
|
Organic mobile phase |
methanol +0.5% acetic acid +0.1% formic acid |
|
Column temperature: |
40 °C |
|
Sample temperature: |
15 °C |
|
Injection volume: |
5 µL |
|
Flow rate: |
0.40 mL/min |
|
Needle: |
FTN 15 μL |
|
Needle wash solvent |
water:methanol:acetonitrile: isopropanol: acetone 20:20:20:20 + 1% formic acid (volumetrically) |
|
Seal wash solvent: |
water: acetonitrile 80:20 (v/v) |
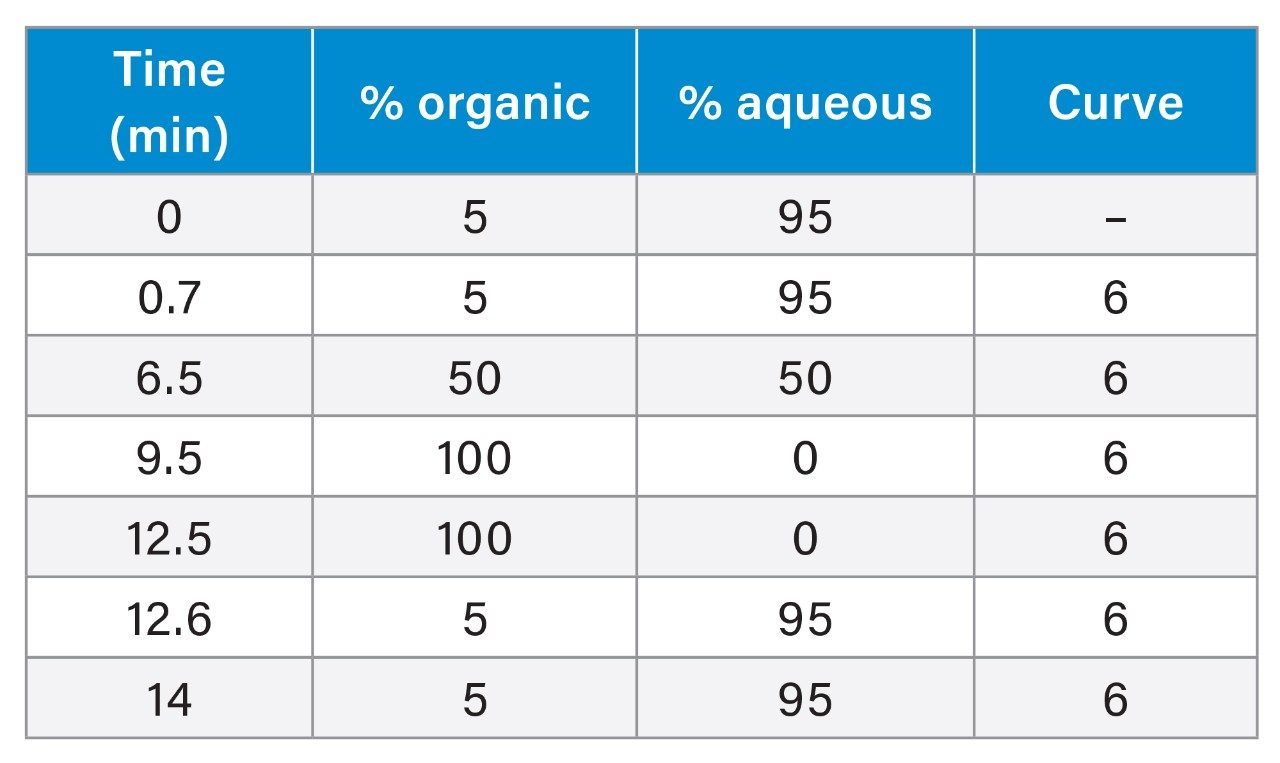
|
System: |
Xevo TQ-S cronos |
|
Ionization mode: |
ESI+ |
|
Acquisition mode: |
Multiple Reaction Monitoring (MRM) |
|
Capillary voltage: |
+0.75 kV |
|
Cone gas flow: |
50 L/Hr |
|
Desolvation temperature: |
600 °C |
|
Desolvation gas flow: |
1100 L/Hr |
|
Source temperature: |
150 °C |
|
Resolution: |
MS1 Unit, MS2 Unit |
|
Software: |
MassLynx v4.2 (TargetLynx XS was used for data processing) |
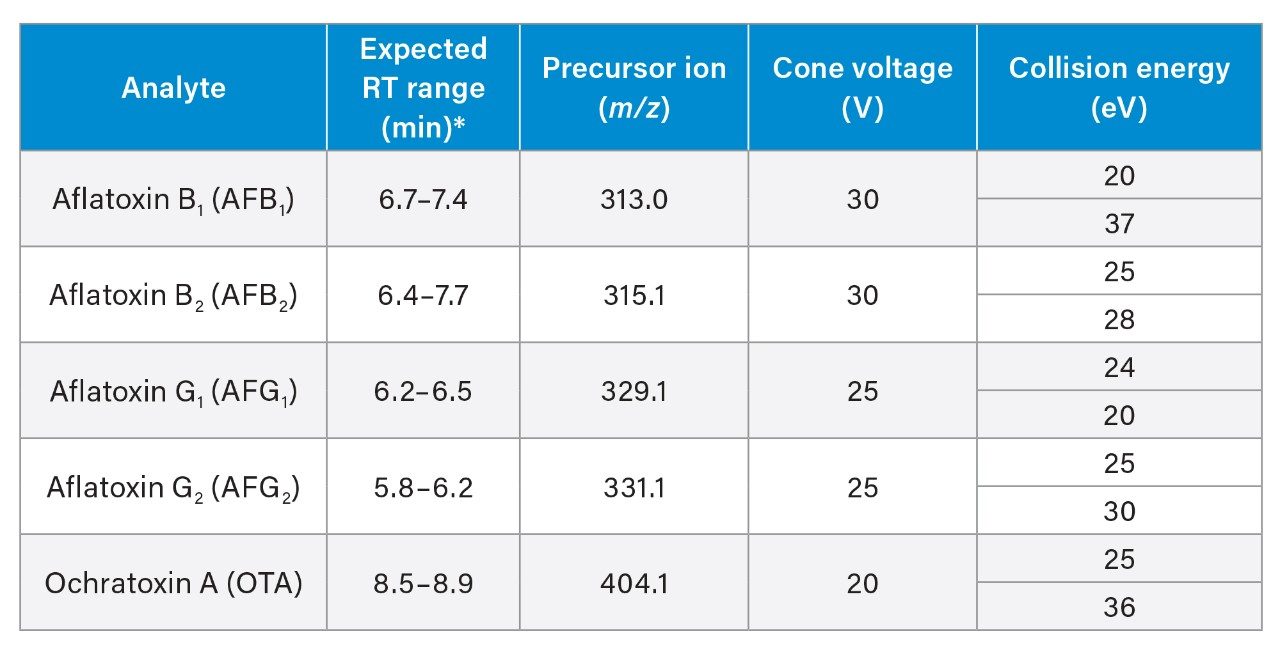
Acetonitrile standards of aflatoxins (mix of AFB1, AFB2, AFG1, and AFG2) at 1 µg/mL each, and OTA at 10 µg/mL were purchased from Biopure (Romer Labs Division Holding GmbH). A working solution was prepared by mixing 250 µL of aflatoxins stock with 100 µL of OTA stock and made to a total volume of 4 mL in a silanized glass amber vial. Serial dilutions of the working solution were prepared prior to analysis, directly into LC vials. 95:5 H2O:MeCN (v/v) was used as diluent for all standards. The range of concentrations was 0.013–5.0 ng/mL for each aflatoxin; and 0.050–20 ng/mL for OTA.
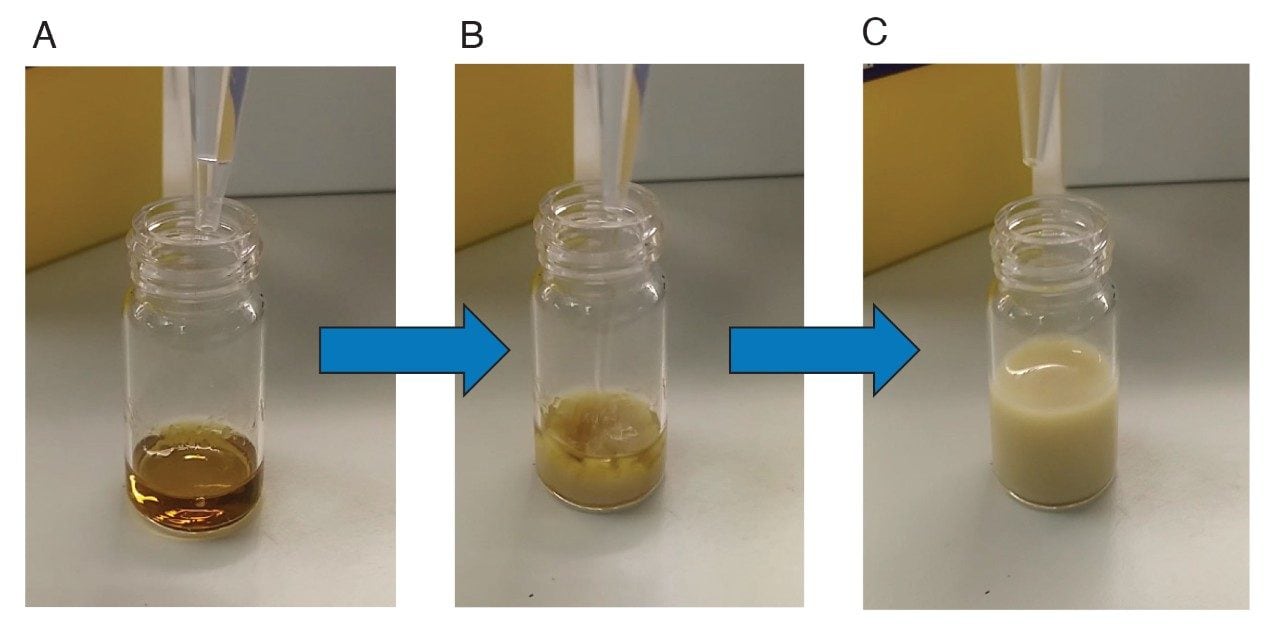
After dilution, the resulting solution turns into a cloudy and viscous suspension with the appearance of a dark precipitate. This is due to the presence of oleoresins that are co-extracted by the 80:20 organic:aqueous mix. Oleoresins and similar lipophilic substances can incapsulate bioactive compounds, such as mycotoxins, thus decreasing the interaction efficiency antigen-antibody when passing the suspension through IAC. Filtration and/or centrifugation of the diluted extract help making the solution clearer, however, they do not improve recoveries of mycotoxins. The incorporation of a defatting step, consisting in a liquid-liquid partition of the supernatant with hexane or equivalent apolar solvent, can decrease the concentration of lipophilic substances prior to dilution. However, this step mitigates yet does not solve problem, and it represents a time-consuming and non-optimal option.
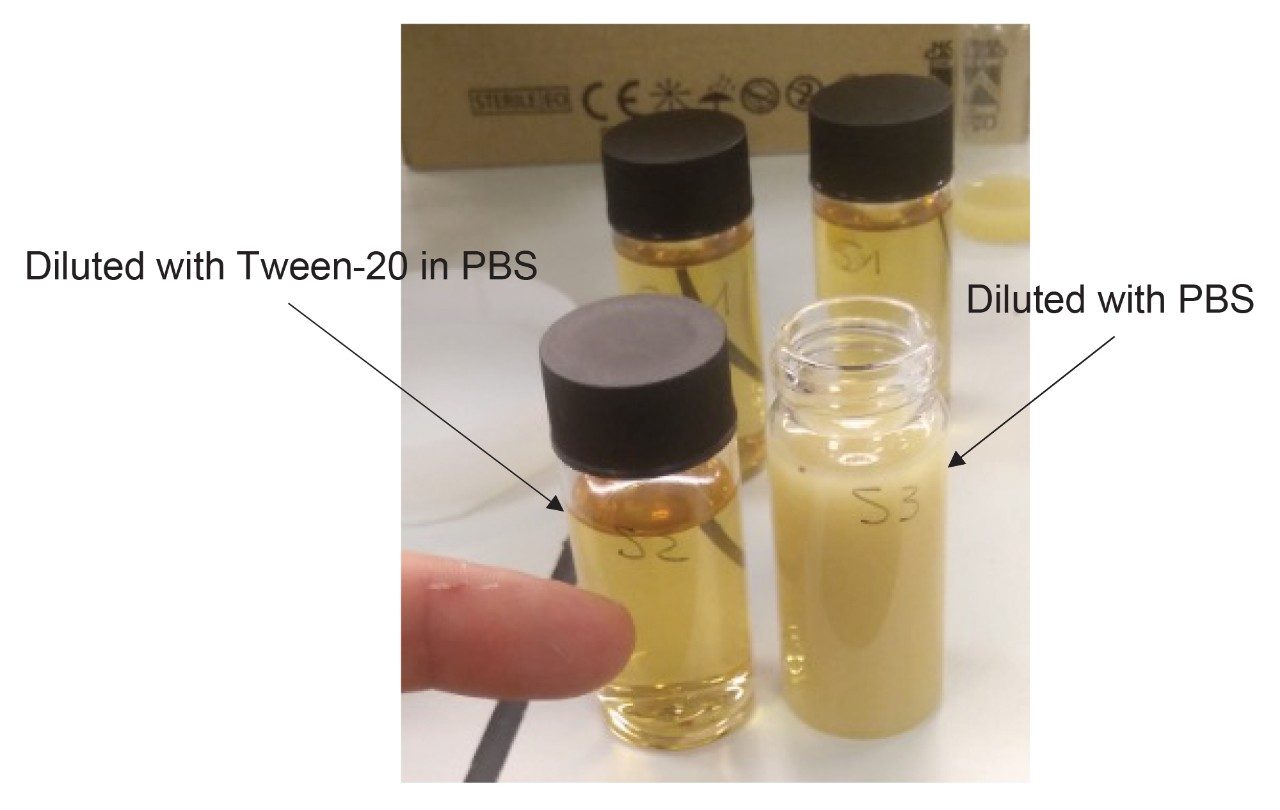
It was found that an effective alternative is to dilute the extract 1:20 (v/v) with a surfactant solution. In particular, a 2% (w/v) Tween-20 in PBS was used to keep the mycotoxins in solution. Also, increasing the dilution factor was important to allow oleoresins to spread in the higher liquid volume by the surfactant. Figure 8 illustrates the comparison between the diluted extract when using PBS only or Tween-20 in PBS. This strategy can also be applied to nutmeg and other spices,13 which are well known to be complex matrices, due to the presence of different types of co-extractive compounds that can interfere with the analytes and give rise to remarkable matrix effects.
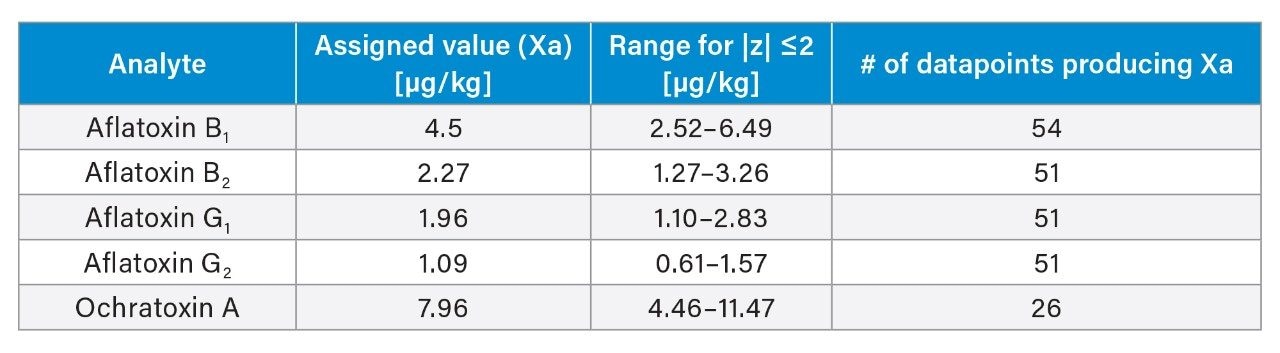
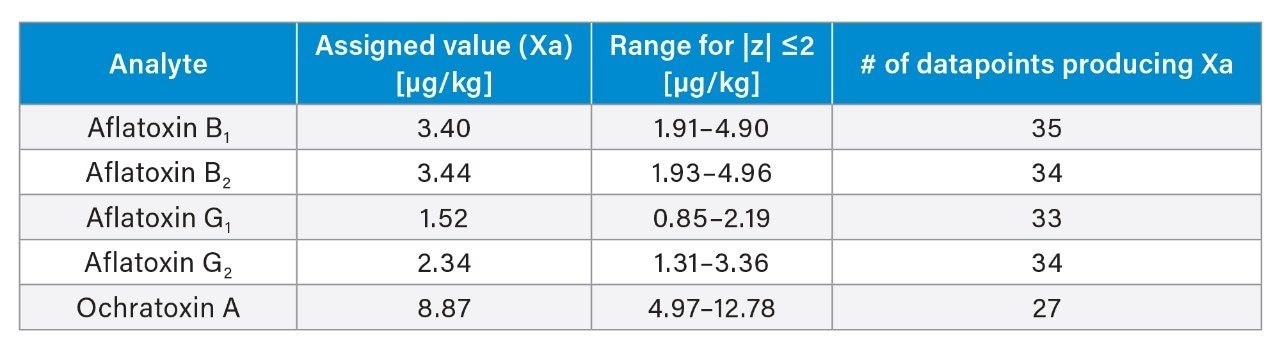
The assigned values have been derived from the consensus of laboratories taking part in a proficiency test, using a variety of methods. The range for for |z| ≤2 is the concentration range within the limits of ±2 z-scores.
PBS is used for diluting the extract and allow to stabilize the pH at 7.0. VICAM Provides 10x concentrate PBS (p/n G1113), which can be diluted 1:10 and ready to be used. Alternatively, it is possible to prepare 10x concentrate PBS as follows:
Dissolve 8.0 g NaCl, 1.2 g Na2HPO4, 0.2 g KH2PO4 and 0.2 g KCl in 990 mL of purified water and adjust to pH 7.0 with concentrated HCl. Bring to 1 L with purified water.
720007298, June 2021