The ACQUITY™ RDa™ Detector Combined With RemoteAnalyzer® Software to Give Simple, Rapid Accurate Mass Information in a Walk-Up Environment
Abstract
In this application note we demonstrate the use of the ACQUITY RDa Detector in combination with the browser-based RemoteAnalyzer software (SpectralWorks - Runcorn, UK) as a simple and intuitive platform enabling accurate mass measurements for synthetic chemists in a walk-up environment.
Benefits
- Routine access to accurate mass measurements for mass confirmation of API (Active Pharmaceutical Ingredient) and related impurities for synthetic chemists.
- Fast scanning rate of the RDa Tof (Time-of-Flight) detector enabling rapid UPLC™ gradients without compromising qualitative data.
- Rapid results via email for convenient, timely decision making on reaction outcomes.
- Flexible RemoteAnalyzer software can be configured to have specific access as role requires
Introduction
Many organic chemists employ mass spectrometry (MS) as a convenient verification tool for their product, and any impurities present in a synthetic reaction.
Single quadrupole mass spectrometers provide only nominal mass data which while useful, does not eliminate the potential of mis-assignment of the target compound, degradants, impurities, or side reaction products. Increased confidence through compound characterization is possible through the accurate mass measurements provided by ToF high resolution mass spectrometry (HRMS) which can require high levels of expertise to operate.
The ACQUITY RDa Detector with its simple, automatic setup was designed to make accurate mass measurements more accessible for a wider range of chemists.
The intuitive ‘point and click’ browser based RemoteAnalyzer software further simplifies sample submission for both single and batch analyses.
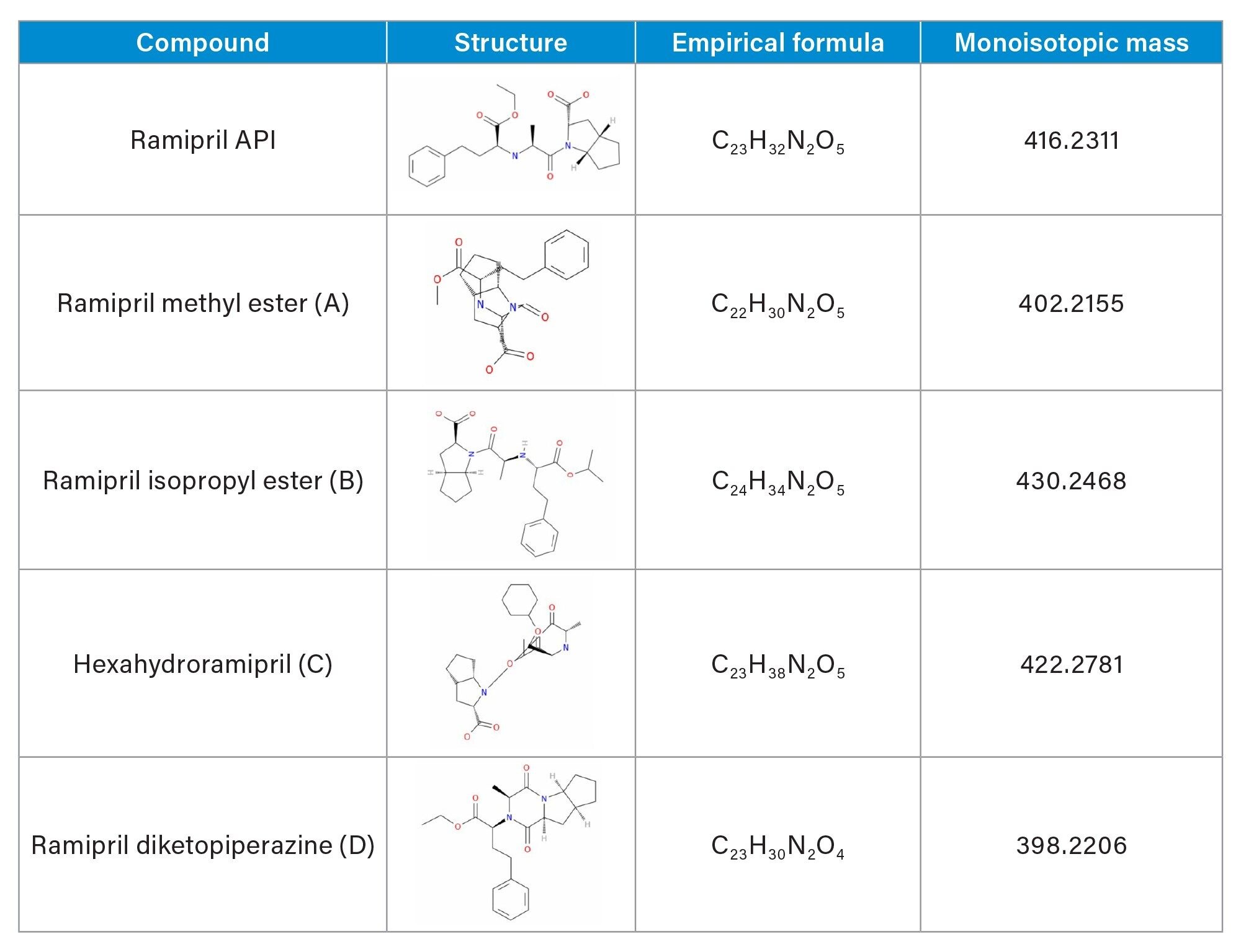
To highlight the benefits of this approach, ramipril 2-[N-[(S)-1-Ethoxycarbonyl-3-phenylpropyl]-Lalanyl]-(1S, 3S, 5S)-2-azabicyclo[3.3.0]octane-3-carboxylic acid (CAS no. 87333-19-5), an angiotensin-converting enzyme (ACE) inhibitor was spiked at 0.1% (w/w) with four related impurities; ramipril isopropyl ester, ramipril diketopiperazine, ramipril methyl ester and hexahydroramipril labelled A-D respectively and analyzed using the ACQUITY RDa.
(ACE) inhibitors constitute an important class of therapeutic agents for the regulation of hypertension.
The chemical structures of ramipril and its potential related impurites are shown in Table 1.
Sample submission and processing was controlled by RemoteAnalyzer software, while data acquisition was carried out by UNIFI with the acquired raw data remaining intact and independent of the RemoteAnalyzer processing.
Experimental
Sample Description
A sample of the ramipril was spiked with four related impurities A-D at levels of 0.1% w/w. All standards were European Pharmacopeial standards and sourced from Merck (Dorset, UK).
A 1 mg/mL solution of ramipril was prepared in methanol along with separate preparations of impurities A-D at concentrations of 200 µg/mL. 980 µL of the ramipril solution was transferred to a vial along with 5 µl of each impurity solution to give a 0.1% (w/w) impurity solution with respect to the API. 100 µl of this solution was transferred to a vial and diluted with 900 µl methanol :water. 80:20.
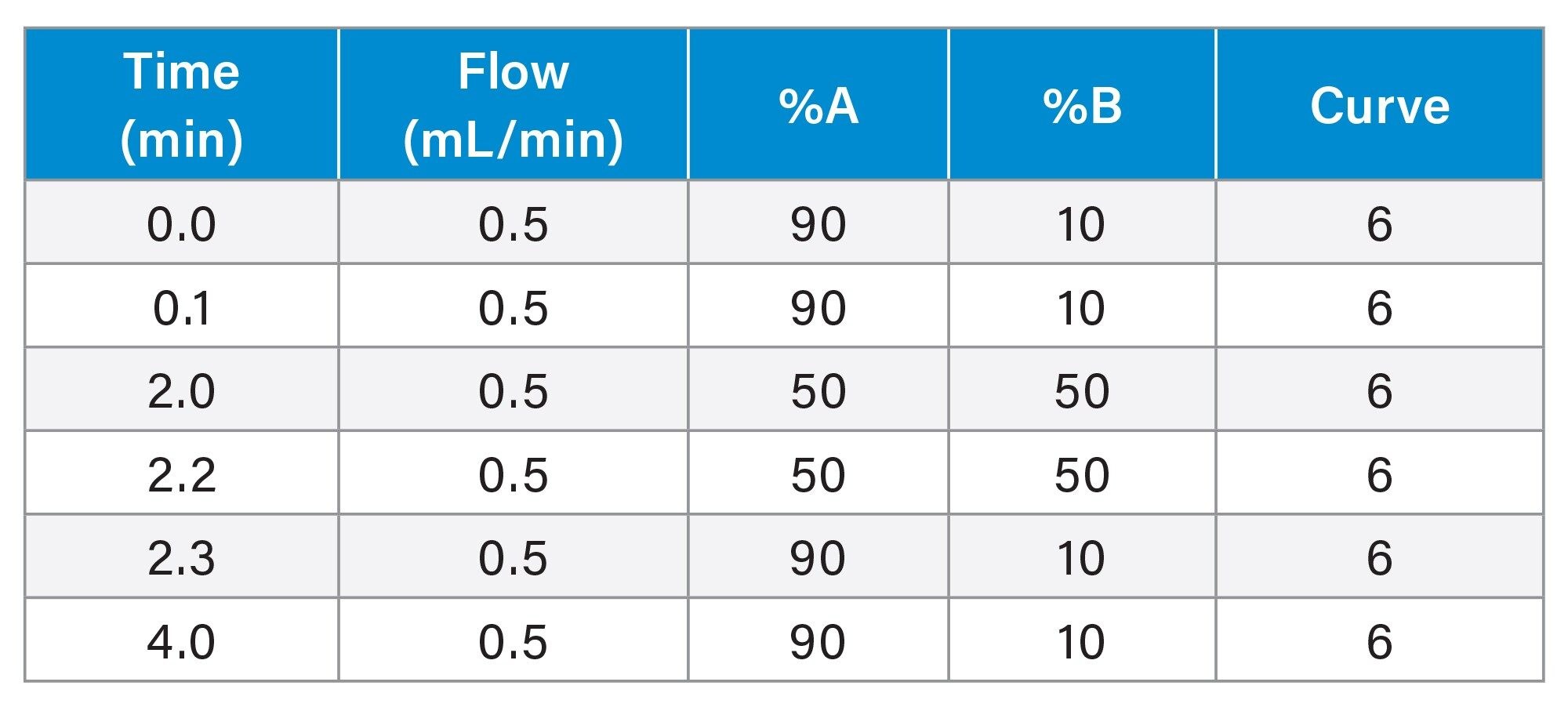
Analysis was carried out using an ACQUITY UPLC I-Class PLUS Binary System with a simple gradient over a four minute runtime. Figure 1, Summarizes the impurity-spiked ramipril sample was submitted for analysis through the RemoteAnalyzer browser following the workflow.
LC Conditions
|
LC system: |
ACQUITY™ UPLC™ I-Class PLUS |
|
Vials: |
TruView Max Recovery Vials, (p/n:186005668CV) |
|
Column(s): |
ACQUITY™ UPLC™ BEH™ C18 50 x 2.1mm,1.7µm (p/n:186002350) |
|
Column temperature: |
80 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
1 µL |
|
Flow rate: |
0.5 mL/min |
|
Mobile phase A: |
2 mM Ammonium formate + 0.1% formic acid |
|
Mobile phase B: |
Acetonitrile + 0.1% formic acid |
Gradient Table
MS Conditions
|
MS system: |
ACQUITY RDa Detector |
|
Ionization mode: |
Positive |
|
Acquisition range: |
50–2000 m/z |
|
Capillary voltage: |
1.5 kV (default) |
|
Cone voltage: |
30 V |
Data Management
|
MS software: |
UNIFI™ 2.1.2.14 RemoteAnalyser®SpectralWorks Ltd. Version 4.56.0.3447 |
|
Informatics: |
waters_connect™ |
Results and Discussion
The ACQUITY RDa Detector was set up automatically, including detector, auto-tune, and mass calibration. Following this routine set up, MS full scan accurate mass data was acquired at a capillary voltage of 1.5 kV and a cone voltage of 30 V.
Initial chromatographic conditions using 40 °C column temperature exhibited very broad, co-eluting peaks at around two minutes retention time. Experimenting with both protic and aprotic solvents did not mitigate the poor chromatography however increasing the column temperature to 80 °C significantly improved peak shape and resolution for all compounds detected. The selection of the ethylene bridged particle column technology ensured running at these temperatures would not adversely impact the column longevity under acidic conditions.1
On submission of the sample, an email was sent to confirm the analysis request was successfully received. On completion of the sample analysis an email was received confirming successful completion of the analysis Workflow steps 1–6.


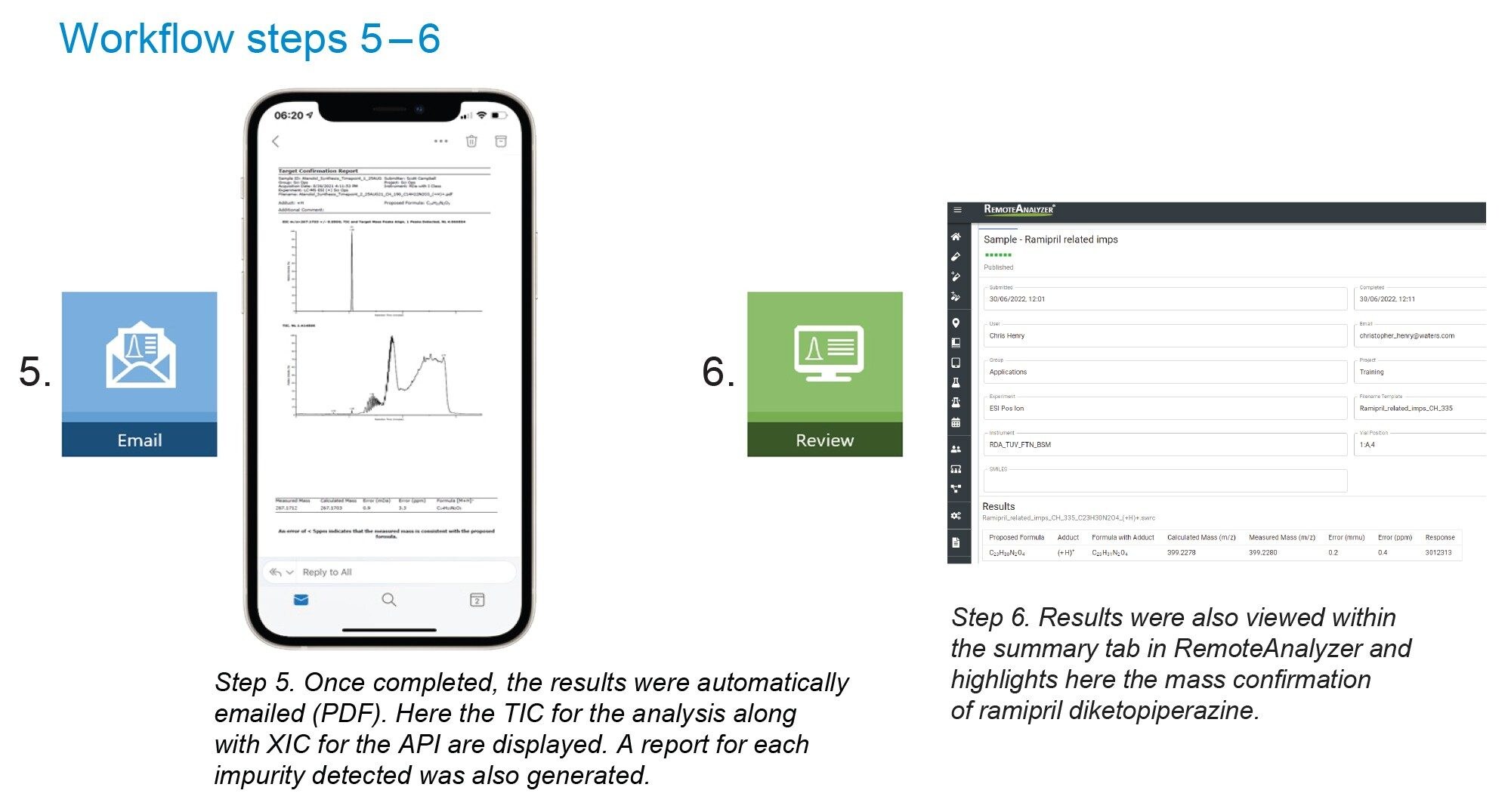
The emailed result contained a pdf document for every proposed formula, Figure 2. Also, a hyperlink (highlighted) was present in the email which when clicked takes the analyst to a sample review page within the RemoteAnalyzer software. This contained copies of the pdf’s along with links to .swrc. files (RemoteAnalyzer propriety file format) for each component detected. When .swrc files were selected, it opened an interactive results page with ‘zoom enabled’ TIC (total Ion Chromatogram along with EIC’s (Extracted Ion Chromatograms) and extracted spectrum for each analyte, Figure 3.
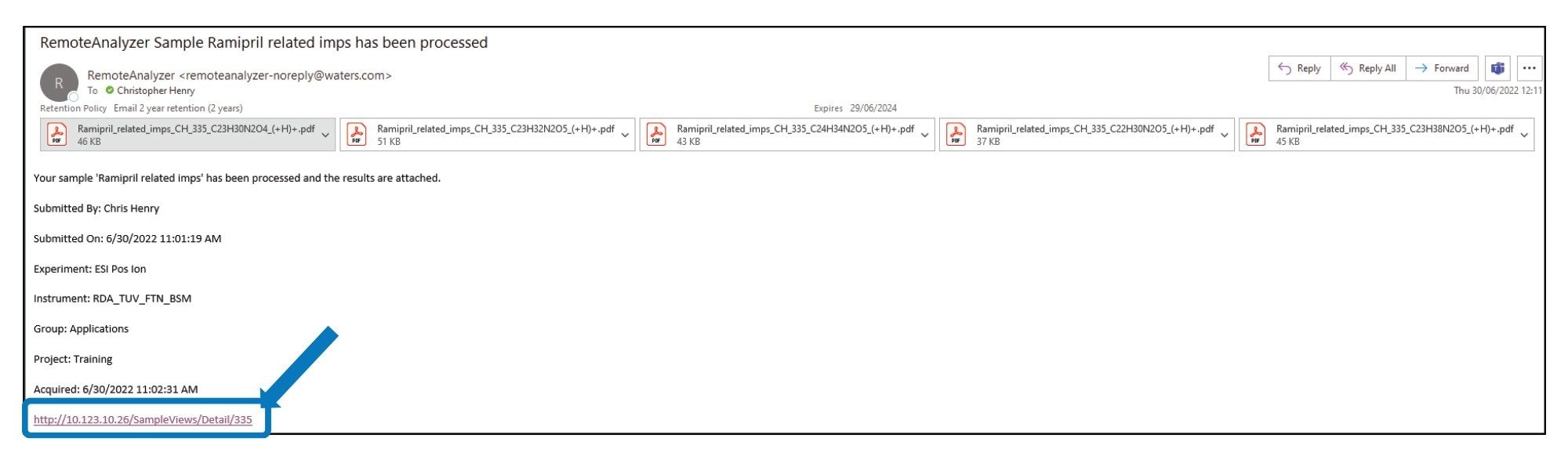
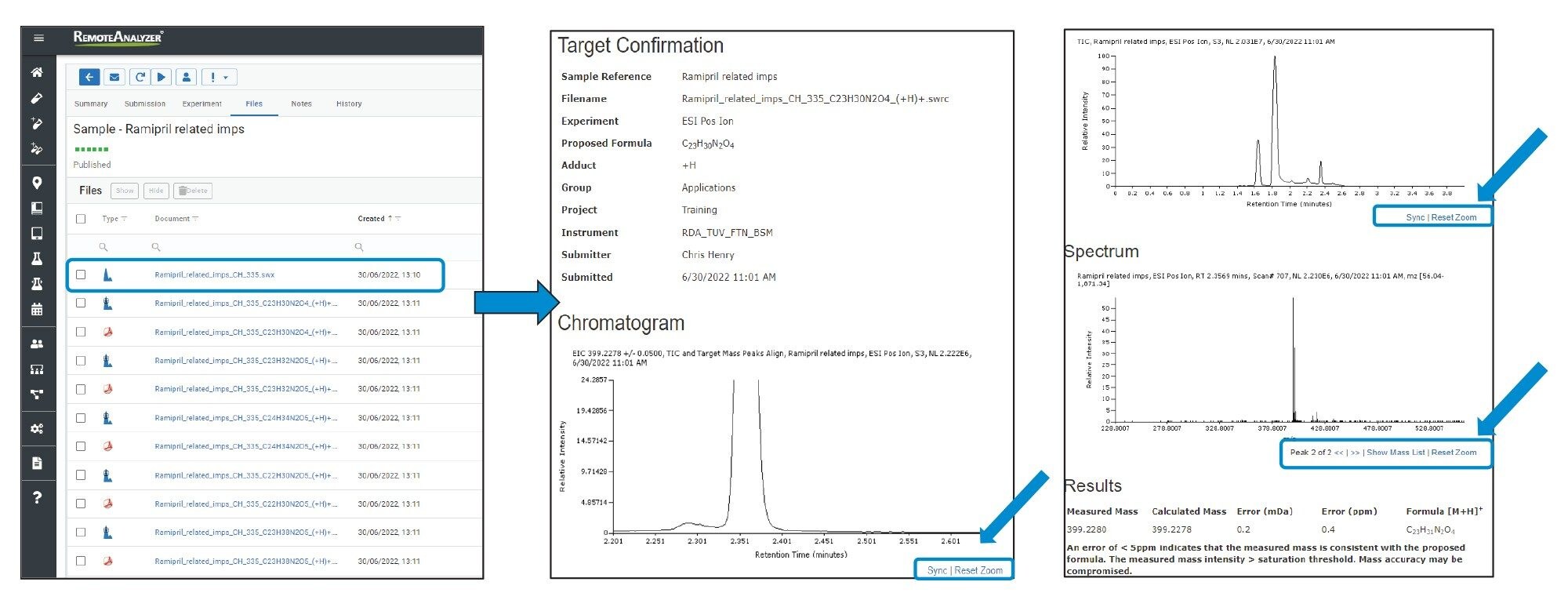
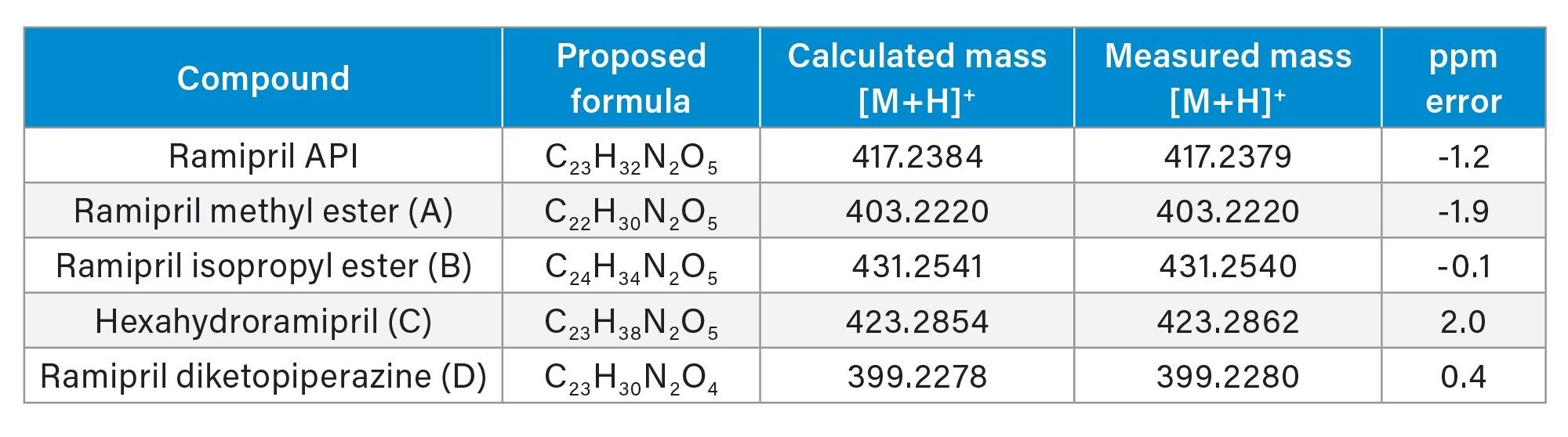
Figure 4 shows a TIC (Total Ion Chromatogram) taken from the RemoteAnalyzer software and the chromatography achieved. The report generated by RemoteAnalyzer has been summarized in Table 2 showing excellent ppm error for all compounds i.e., between -0.1 and 2 ppm for all compounds.
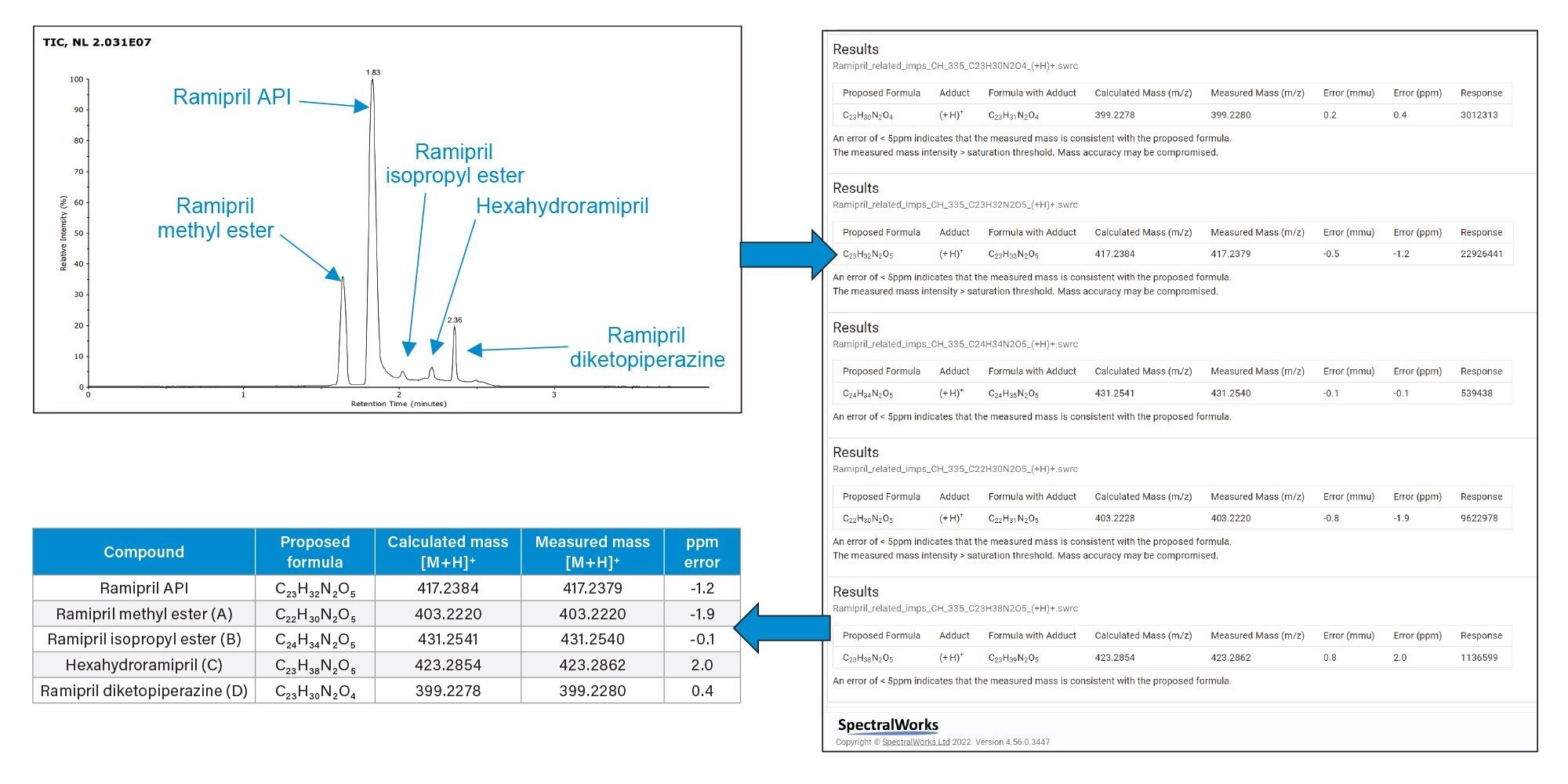
The chromatography shown slight co-elution of the isopropyl ester impurity and the API however due to the fast scan speed of full scan time of flight detection allows for the accurate quantitative integrations and assignment of closely eluting peaks.2
Conclusion
Combining the ACQUITY RDa Detector with the RemoteAnalyzer software provides routine access to accurate mass measurements for synthetic chemists.
Ramipril and the four spiked related substances were all detected with excellent mass accuracies of ≤2 ppm using a 4 minute UPLC run time.
The fast scan rate RDa Detector enables the use of rapid UPLC gradients without compromising qualitative mass measurements for efficient, reliable sample turnaround time.
With sample submission and processing controlled by the browser based RemoteAnalyzer software, chemists can retrieve and review data remotely from anywhere without needing to return to the instrument controlling PC.
References
- A Review of Waters Hybrid Particle Technology. Part 2: Ethylene Bridged [BEH Technology™] Hybrids and Their Use in Liquid Chromatography.
- Alelyunas YW, Wrona MD, Cook K, McDonald S, Rainville P: Effect of MS Scan Speed on UPLC Peak Separation and Metabolite Identification: Time-of-Flight HRMS v Orbitrap.
720007690, May 2025