Analysis of Amino Acid Content in Commercially Available Supplements Using an Updated HPLC Method
Abstract
With the increasing popularity of commercially available supplements, there is a need to monitor amino acid content in these products to ensure both product safety and efficacy. However, these products are controlled by varying regulatory agencies from country to country, each with different safety requirements.1,2 While the medicinal properties of amino acids in supplements is unknown, the label claim of these supplements is assumed to be accurate. To verify the accuracy of label claims, two natural supplement product samples were evaluated using an updated HPLC method.4
Benefits
- Ability to quantitate amino acids in dietary supplement samples
- Using the AccQ•Tag Ultra™ C18 2.5 µm Column enables a shorter run time compared to legacy HPLC methods resulting in higher throughput, and lower solvent consumption
Introduction
Amino acid analysis using pre-column derivatization methods is performed regularly in a wide range of applications. More recently, the analysis of amino acids has also become increasingly popular for analysis of dietary supplements because of their widespread sale and use. Analysis of dietary supplements can be challenging for a variety of reasons, starting with the fact that they can come in many different forms, each with different solubility properties. Finally, flavorings and other stabilizers may or may not derivatize, which can impact amino acid analysis. To quantify amino acids in these supplements, pre-column derivatization methods are often of value to minimize the interference of other components present in the formulation that may impact quantitation. The pre-column derivatization used for this work is 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC reagent). The reaction is carried out in an 80% aqueous environment which minimizes interferences from salts and other compounds in the sample matrix.
To achieve accurate and reliable results, it is important to have good methodologies for quantitating amino acids in supplement samples. The methodology showcased in this work allows for reliable quantitation of amino acids in supplement samples ranging from powdered drink mixes to tablets.
Experimental
All calibration standards were prepared from Waters™ Amino Acid Standard (p/n: WAT088122), and Waters™ Cell Culture Standard Kit (p/n: 186009300) using norvaline (p/n: 186009301) as the internal standard and 0.1 N HCl as the diluent.3 The internal standard stock was prepared at 2500 µM in 0.1 N HCl. The final concentration of the calibrants varied to match the content range of each supplement, and a concentration of 250 µM was used for norvaline (internal standard).
Amino Acid Supplement Sample Preparation
The purchased samples consisted of 1 amino acid supplement tablet, Sample 1, and 1 amino acid supplement powdered drink mix, Sample 2.
The samples were prepared as follows:
Sample 1 (amino acid supplement tablet) was crushed using a mortar and pestle and diluted in 10 mL of 0.1 N HCl in water. Sample 1 was then placed onto a shaker for 30 mins followed by centrifugation at 2000 rpm for 10 mins. The supernatant was subsequently transferred to a clean centrifuge tube and diluted 1:10 in 0.1 N HCl in water.
Sample 2 (Powdered Drink Mix Supplement) was reconstituted using the recommended serving size of liquid listed in per scoop weight, which was weighed at 7.160 g in 0.1 N HCl in water. The sample was stirred for 30 minutes followed by centrifugation at 2000 rpm for 10 mins. The supernatant was then transferred to a clean centrifuge tube and diluted at 1:100 in 0.1 N HCl in water.
Both samples were then derivatized using Waters AccQ•Tag Ultra derivatization protocol.
LC Conditions
|
LC systems: |
ACQUITY Arc™ System with CH-A column heater |
|
Detection: |
ACQUITY Arc™ - 2489 UV Detector with low dispersion 10 mm UHPLC flow cell (p/n: 176017007) |
|
Wavelength: |
260 nm |
|
Sampling rate: |
10 Hz |
|
Vials: |
LCGC certified clear glass 12 x 32 mm screw neck vial, total recovery with cap and PTFE/Silicone septum (not pre-slit) (p/n: 186000384C) |
|
Column(s): |
AccQ•Tag™ Ultra C18, 2.5 µm 4.6 x 150 mm (p/n: 186010407) |
|
Column temperature for Hydrolysate and Cell Culture Standard: |
43 °C |
|
Sample temperature: |
20 °C |
|
Injection volume: |
2 µL |
|
Flow rate: |
1.5 mL/min |
|
Mobile phase A: |
AccQ•Tag™ Ultra Eluent A (p/n: 186003838) |
|
Mobile phase B: |
90:10 (v/v) Water:AccQ•Tag Ultra Eluent B |
|
Mobile phase C: |
Milli-Q Water |
|
Mobile phase D: |
AccQ•Tag™ Ultra Eluent B (p/n: 186003839) |
|
Sample manager wash: |
95:5 (v/v) Water:Acetonitrile |
|
Sample manager purge: |
95:5 (v/v) Water:Acetonitrile |
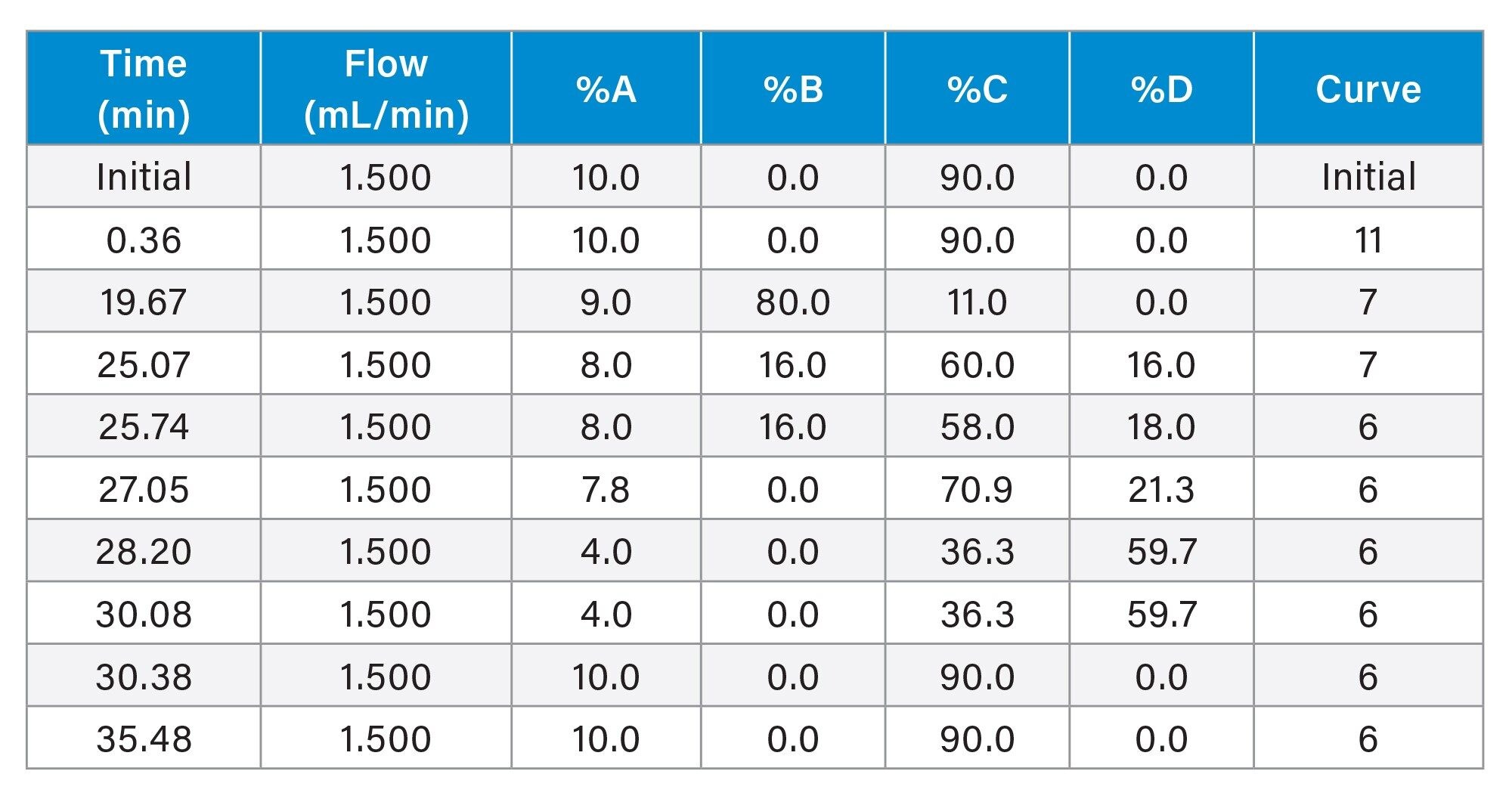
Gradient Table
Data Management
|
Chromatography data system: |
Empower 3.6.1 |
Results and Discussion
Several energy supplements were purchased and analyzed to quantify the amino acid content. Pre-column derivatization was performed using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC), then the samples were analyzed using an HPLC or UHPLC system coupled with UV or Fluorescence detection using reversed-phase liquid chromatography. Results were compared to the label claim of the products.
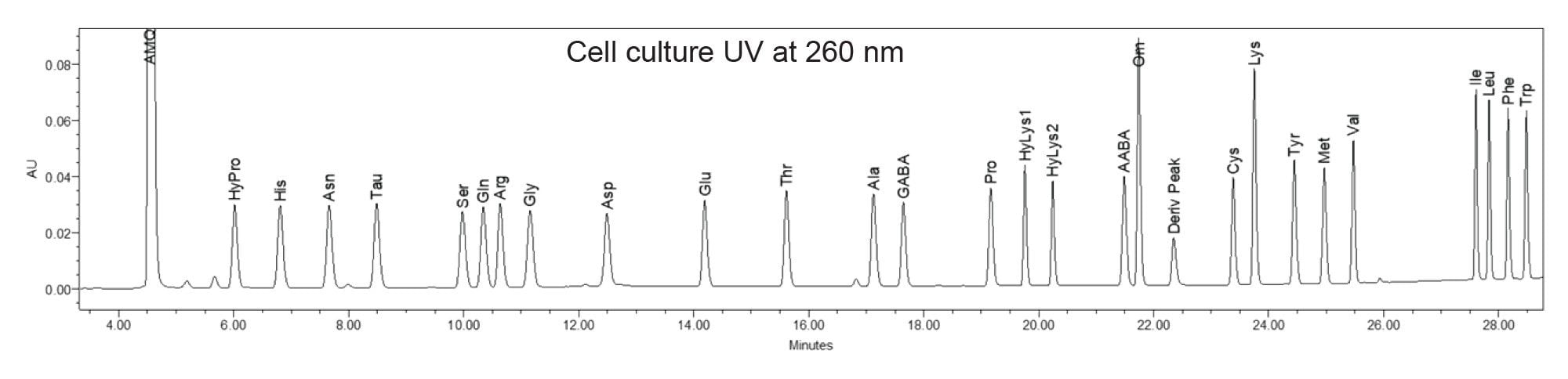
Analysis of amino acids in food and supplements is critical to ensure product safety and accuracy of the product’s label claim. Separation of the amino acids contained in the cell culture standards are shown in Figure 1.
Amino Acid Supplement Sample Results
The total amino acid content for the supplement tablet, Sample 1, was equal to 5000 mg per 5 tablets, which was taken to be equivalent to 1000 mg per 1 tablet. The amino acid content was determined by analysis of a single tablet. The amino acid content per sample was calculated based off of the amino acid weight per injection. Adjustments were made for the dilution of the sample, as weights were summed for total value. The resulting amino acid content was determined to be 1.008 mg per tablet, which is a difference of <1% versus the label claim (Table 1).
Individual amino acid content was provided for the powdered drink mix, Sample 2. Amino acid label claims reported in weighed sample size was based on weight of powder and volume of water for dissolution. Results were converted to weight per amino acid and total amino acid weight of sample (based on total dilution). Listed amino acids were identified and the resulting amount versus the label claims is shown in Table 2.
Results were compared to the label claim of the products, including one powdered drink mix supplement and one amino acid supplement tablet. The results demonstrate the ability to quantify amino acid content in numerous energy supplements.
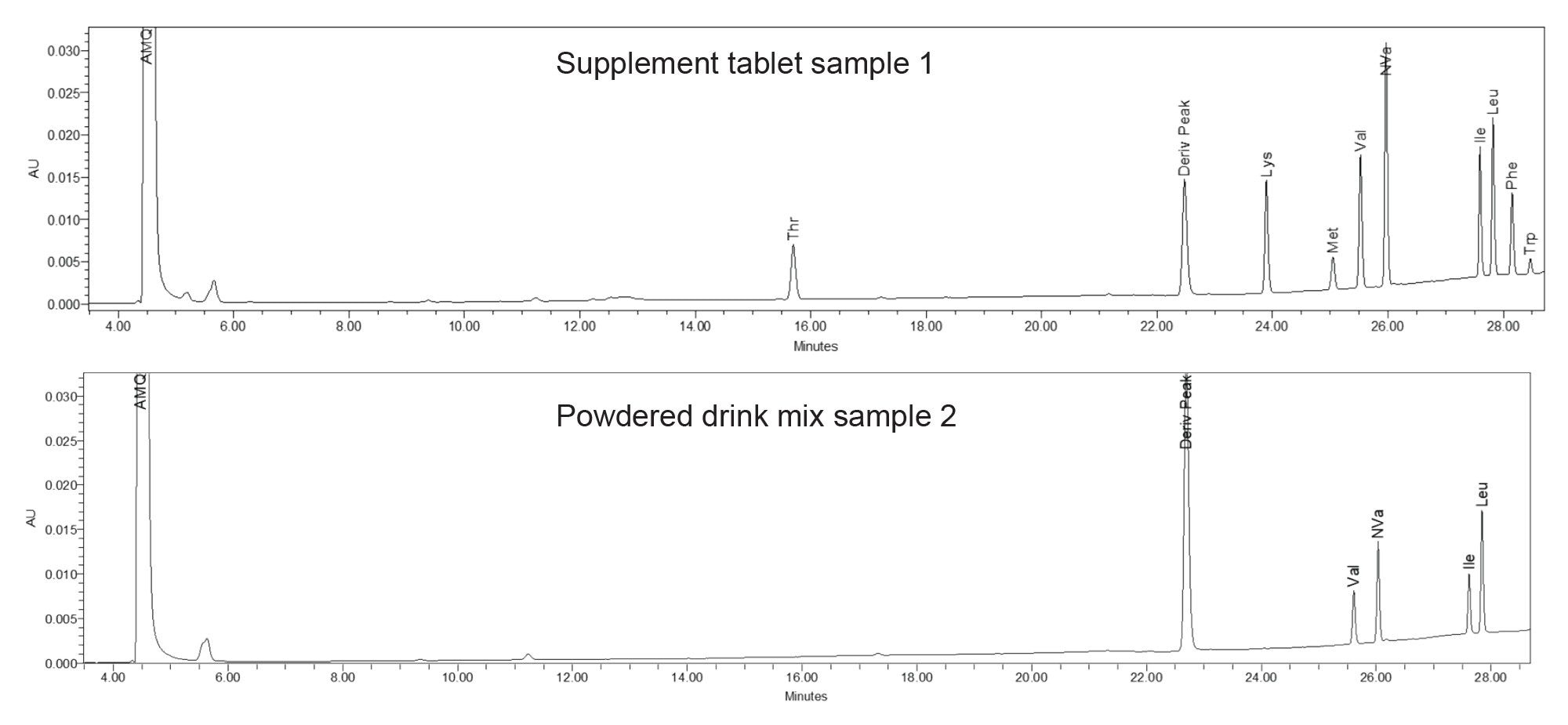
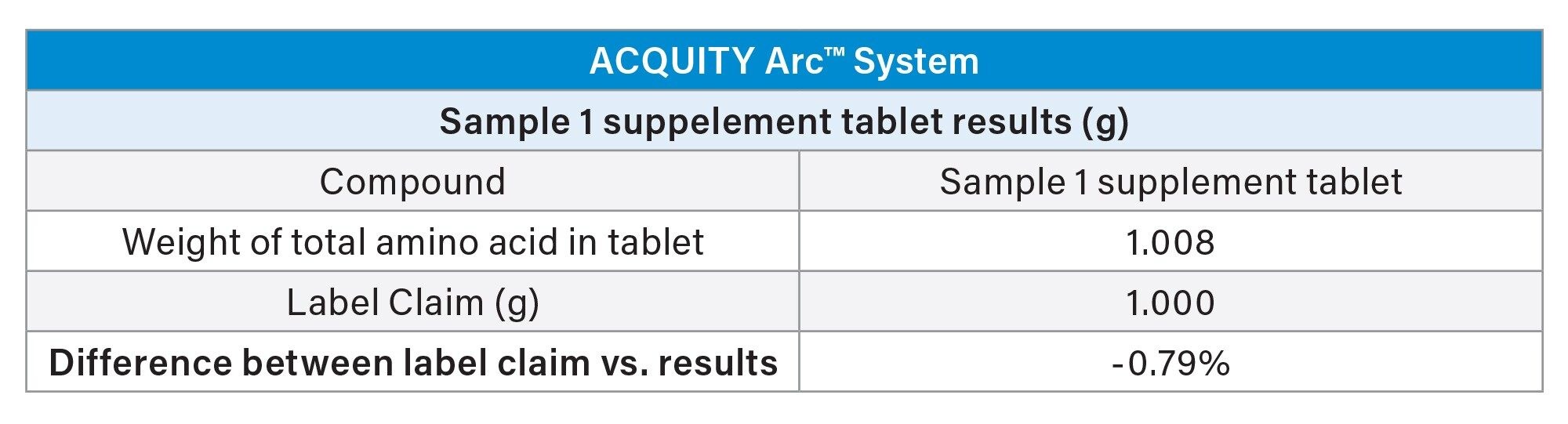
All of the samples were analyzed using the Waters AccQ•Tag Ultra Derivatization kit which minimizes interference in the samples allowing for accurate quantitation. Total weight of amino acids in a single tablet for Sample 1 was found to be within 1% of the label claim.
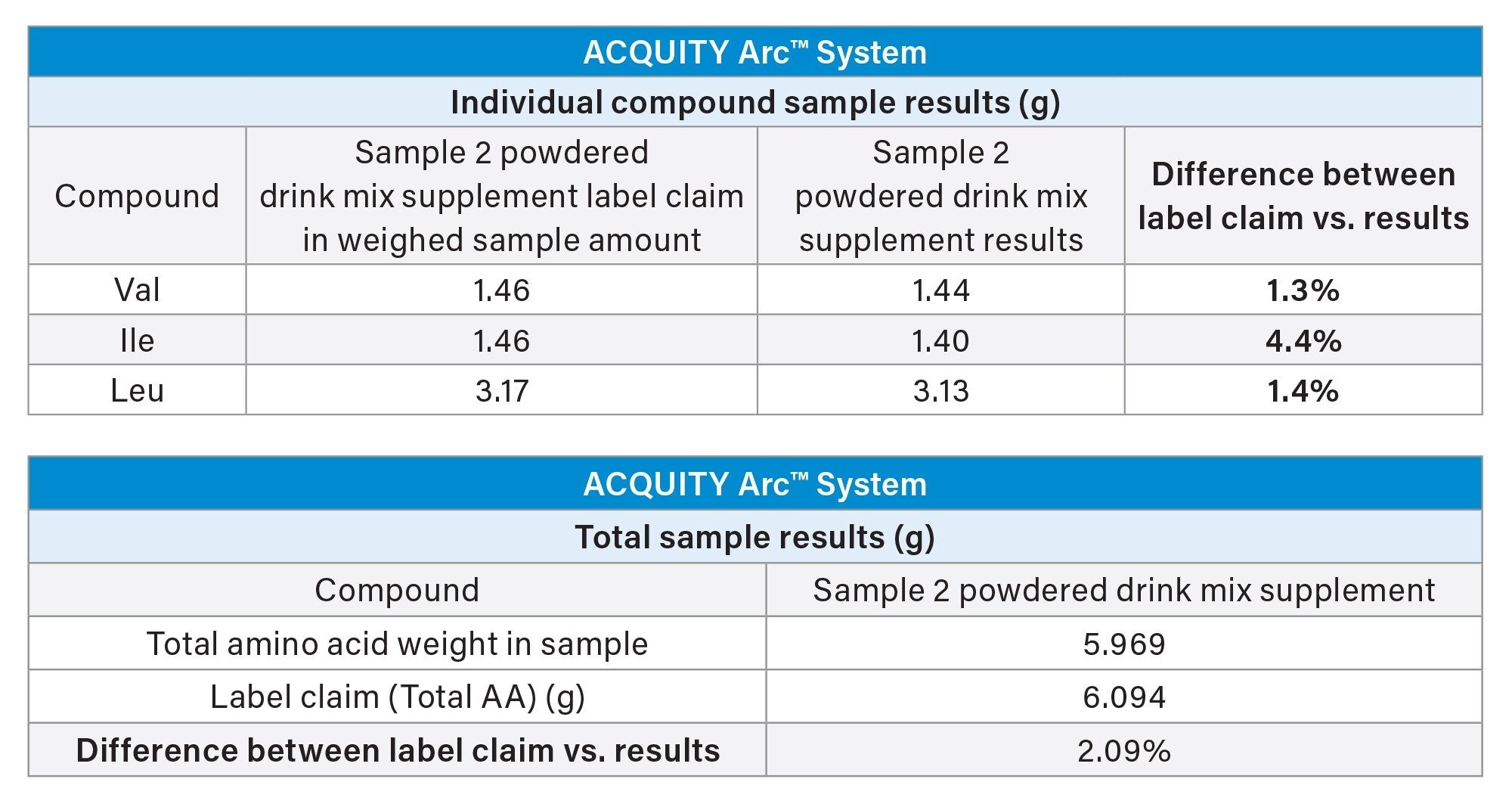
Total weight of amino acids for Sample 2 was found to be within 2.2% of the label claim and individual amino acid content was found to be within 4.5% of label claim.
Conclusion
Analysis of amino acids in food and supplements is critical to ensure product safety and accuracy of the product’s label claim. Quantification of dietary supplements showed good agreement, within 4.5% of the amino acid content, to the label claim of the supplement samples, demonstrating accuracy of the method. The results demonstrate the ability to quantify amino acid content in numerous energy supplements.
References
- Miro Smirga. International Regulations on Amino Acid Use in Foods and Supplement and Recommendations to Control Their Safety Based on Purity and Quality, Journal of Nutrition. 150:2602S-2605S. 2020.
- Ashley Roberts. The Safety and Regulatory Process for Amino Acids in Europe and the United States. Journal of Nutrition. 146(Suppl):2635S-2642S, 2016.
- Amino Acid Standard Kits Care and Use Manual. Waters Care and Use Manual. 72000663EN.
- Kimberly Martin, Kenneth D. Berthelette, Paula Hong. Instrument Considerations for Reliable Amino Acid Analysis Using AccQ•Tag™ Ultra C18 2.5 µm Column. Waters Application Note. 720007678EN. 2022.
- Paula Hong, Donna Johnson, Donald A. Trinite, Bill Warren, and Ning Zhang. Comprehensive Guide to Hydrolysis and Analysis of Amino Acids. Waters Corporation. 2019.
720008053, September 2023