Method Robustness Testing Using Empower™ Method Validation Manager Software Aided by the Empower Sample Set Generator to Automate Method Creation
Abstract
Robustness testing is a measure of a method’s ability to remain unaffected by the minor changes of chromatographic parameters. In this work, robustness of a method for the analysis of naphazoline hydrochloride, pheniramine and associated related substances was investigated using Empower Method Validation Manager (MVM) Software. The robustness multivariable design of experiments (DoE) was created to study the effect of individual parameters and their interaction on the chromatographic resolution between the peaks. The Empower Sample Set Generator (SSG) was used to automatically create a sample set method or injection sequence, instrument methods, and method sets required to perform the entire study in one run. The effect plots generated by the Empower MVM Software clearly showed which parameters have the greatest effect on the method performance and provided knowledge for establishing a set of controls to assure method meets the expected criteria during routine use.
Benefits
- Enhance method understanding and identify acceptable operating conditions through method robustness testing using a multivariable DoE with Empower Method Validation Manager (MVM) Software
- Automate creation of chromatographic methods for robustness testing with Empower Sample Set Generator (SSG) tool
- Identify the effect of method parameters and their interaction on the performance using effects plots generated by the Empower MVM Software
Introduction
Robustness testing is a critical task to demonstrate that the method remains unaffected by the minor changes in chromatographic parameters, providing an indication of its reliability during routine use.1,2 Performing robustness during method development stage should be considered so that the critical parameters affecting method performance can be identified and optimized in the process. The parameters and appropriate associated ranges to be tested experimentally should be selected based on prior knowledge and risk assessment.2 Implementing risk assessment into robustness helps to identify the risk associated with how each parameter impacts the data generated by the method. Based on the outcome of robustness, a control strategy can be established to define method’s acceptable operating ranges, minimize and control source of variability.
Method robustness is typically determined by using either one factor at a time (OFAT) or multivariable approach with a design of experiments (DoE).3 With an OFAT approach, only one factor (or chromatographic parameter) is investigated while others remain unchanged. This is a time-consuming process and often important interactions between variables such as temperature changes with flow rate are not identified. The multivariate DoE approach shows the effect of each independent parameter and multiple parameters simultaneously.
In this work, robustness of a method for the analysis of naphazoline hydrochloride (HCl), pheniramine maleate, and associated related substances was assessed using Empower MVM Software, aided by the Empower SSG to automate method creation. The robustness DoE was created to study the multivariable effect and the effect plots were used to determine which parameters had the most impact on method performance.
Experimental
Compounds (Table 1) were purchased from Sigma-Aldrich and Toronto Research Chemicals (TRC). Mass spectrometry grade reagents and solvents were obtained from Honeywell.
Sample Description
Standard solutions
Individual stock solutions were prepared in methanol at 4.0 mg/mL. Stock solutions were diluted with 80:20 water/methanol diluent to make a mixture standard solution for robustness testing containing naphazoline HCl and pheniramine maleate active ingredients at 0.1 mg/mL and related substances at 10 µg/mL. List of the compounds used in this study is shown in Table 1.
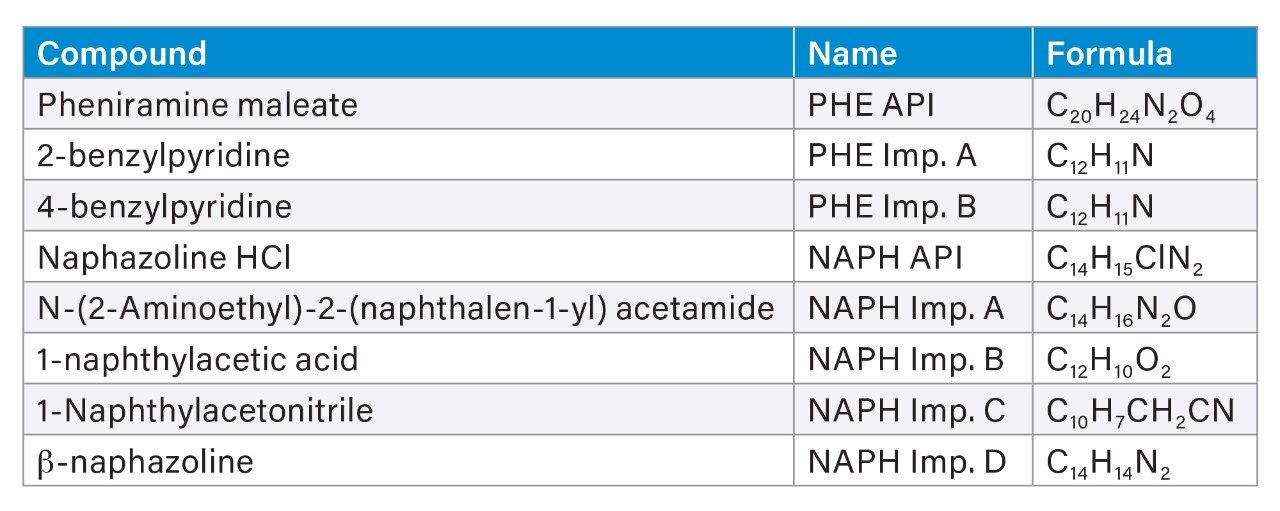
Final method
|
LC system: |
Arc™ Premier System, column manager with active pre-heating, PDA Detector |
|
Vials: |
LCMS Maximum Recovery 2 mL volume, p/n: 600000670CV |
|
Detection: |
UV at 260 nm |
|
Column: |
XSelect™ Premier CSH C18, 4.6 x 150 mm, 2.5 µm (p/n: 186009874) |
|
Column temp.: |
44°C |
|
Sample temp.: |
10°C |
|
Injection volume: |
5.0 µL |
|
Flow rate: |
1.2 mL/min |
|
Mobile phase A: |
0.1% Formic acid in water |
|
Mobile phase B: |
0.1% Formic acid in methanol |
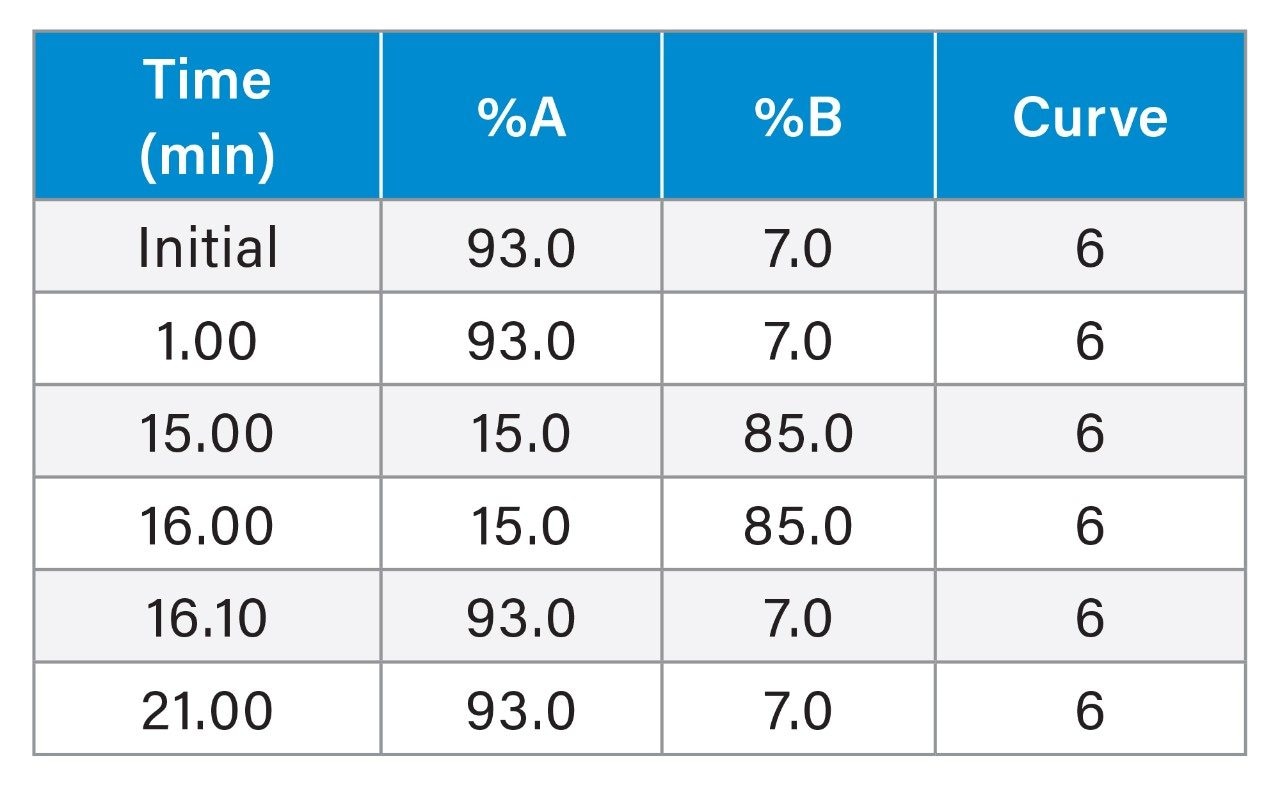
Gradient Table
Data Management
|
Chromatography software: |
Empower 3 Feature Release 5 Service Release 5 (FR5 SR5) |
Results and Discussion
The robustness of a method for the analysis of naphazoline HCl, pheniramine maleate, and associated related substances was conducted by performing a multivariable DoE study, utilizing Empower MVM Software. The chromatographic separation was performed using a XSelect Premier CSH C18 based on previously described method4, with some modifications of the chromatographic conditions. The column temperature, flow rate, composition of the organic solvent at the beginning and end of the gradient were optimized to achieve a robust separation. A representative chromatograph acquired using the final method conditions is shown in Figure 1.
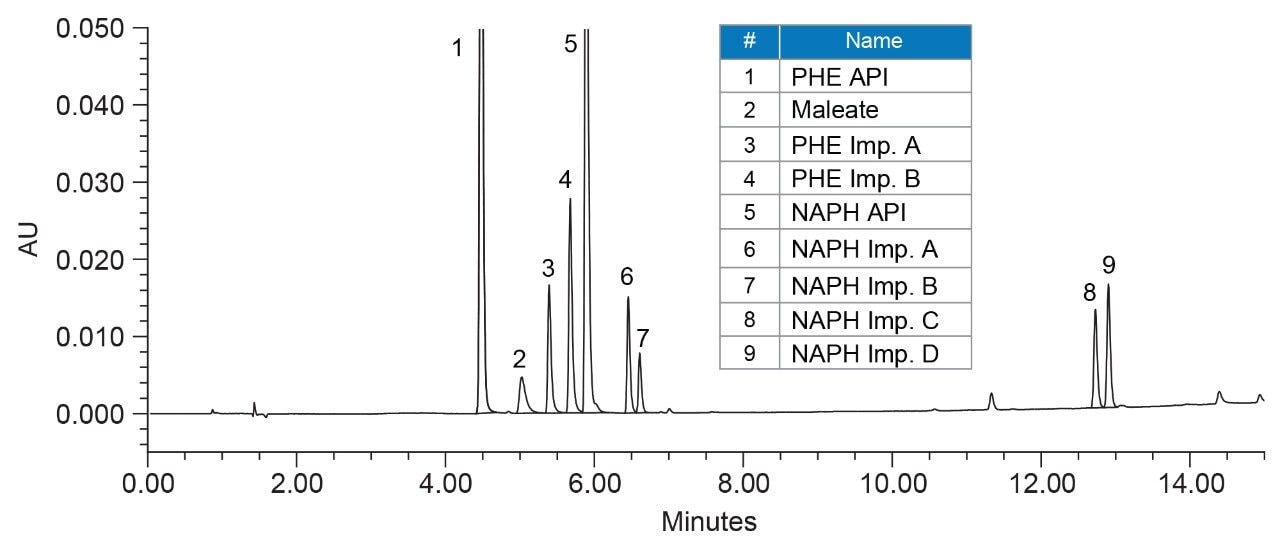
About Empower MVM
The Empower Method Validation Manager (MVM) is a compliant-ready software that automates, streamlines, and simplifies methods validation workflows.4,5 The entire method validation process is performed within a single software application, from creating a validation protocol method to acquiring, reviewing, analyzing, approving, and reporting validation data, including a full complement of statistical results. The validation tests and data are checked during the study for adherence to the validation requirements and acceptance criteria, with secure data storage and audit trails.
About Empower SSG
The Empower Sample Set Generator (SSG) tool automates the creation of instrument methods, method sets, and sample set methods, while varying the chromatographic parameters.5 The Empower method sets and instruments methods are automatically created and structured in the sample set method according to the experiment design as a ready-to-run injection sequence. Automating creation of chromatographic methods minimizes transcription errors that may arise during the manual process and time spent generating methods, providing confidence that all chromatographic runs are completed with correctly created methods.
Robustness study
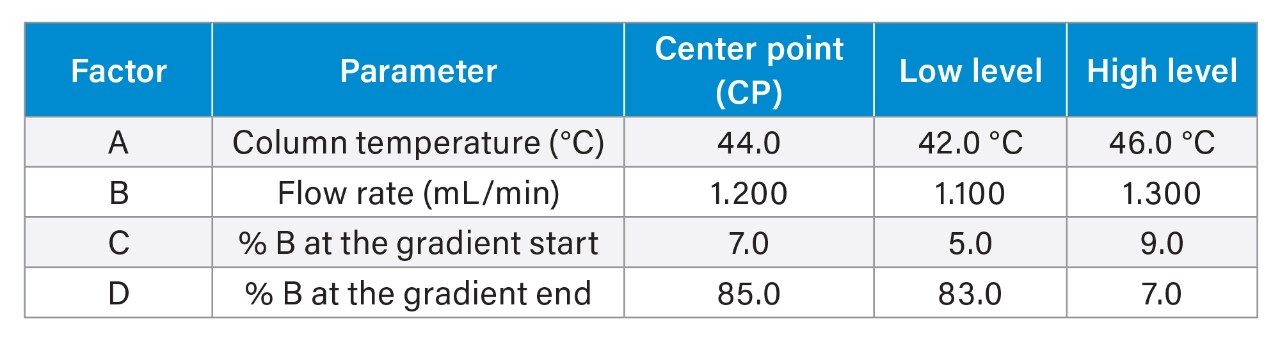
First, in risk assessment, parameters that may have the most impact on method performance were identified based on previous knowledge. The four parameters (factors) were investigated in this work and included column temperature, flow rate, composition (%) of organic solvent at the start and end of the gradient (Table 2). The effect of these parameters and their ranges was investigated experimentally using a robustness test in the Empower MVM Software. A DoE study was created to assess the multivariable effect on the chromatographic resolution between peaks, with acceptance criteria set to a USP Resolution ≥ 1.5. Using a full factorial for four factors with 2 levels (low and high), sixteen design points or experiments with a combination of different instrument conditions (Figure 2) were created for the robustness study.
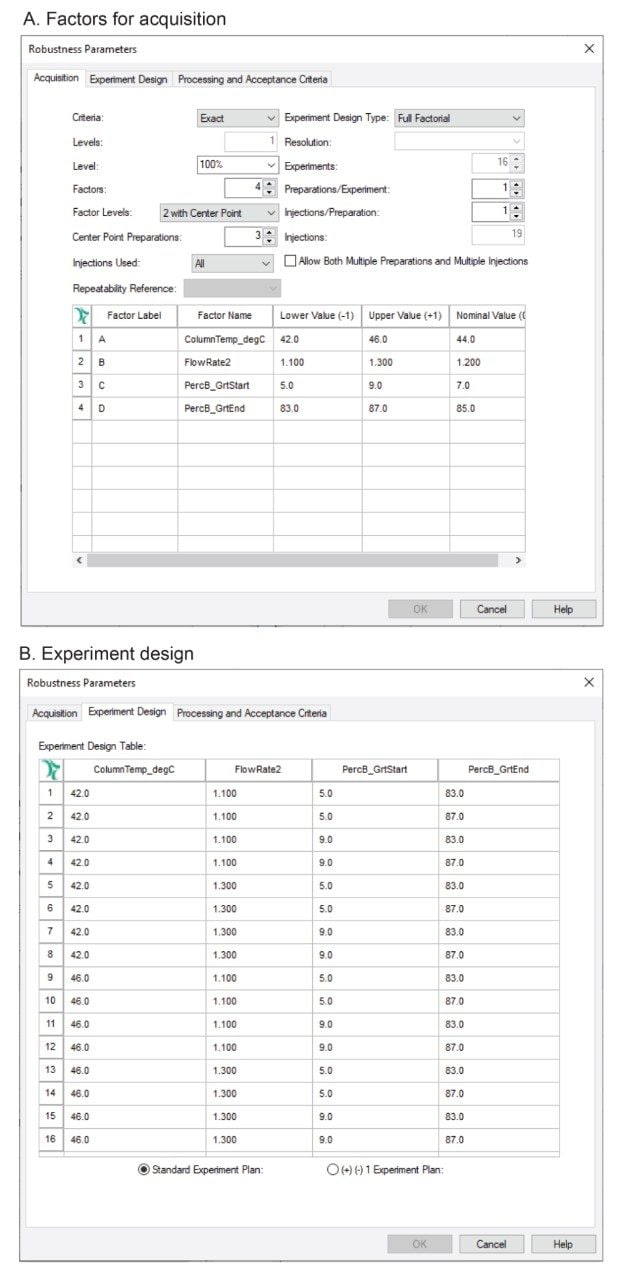
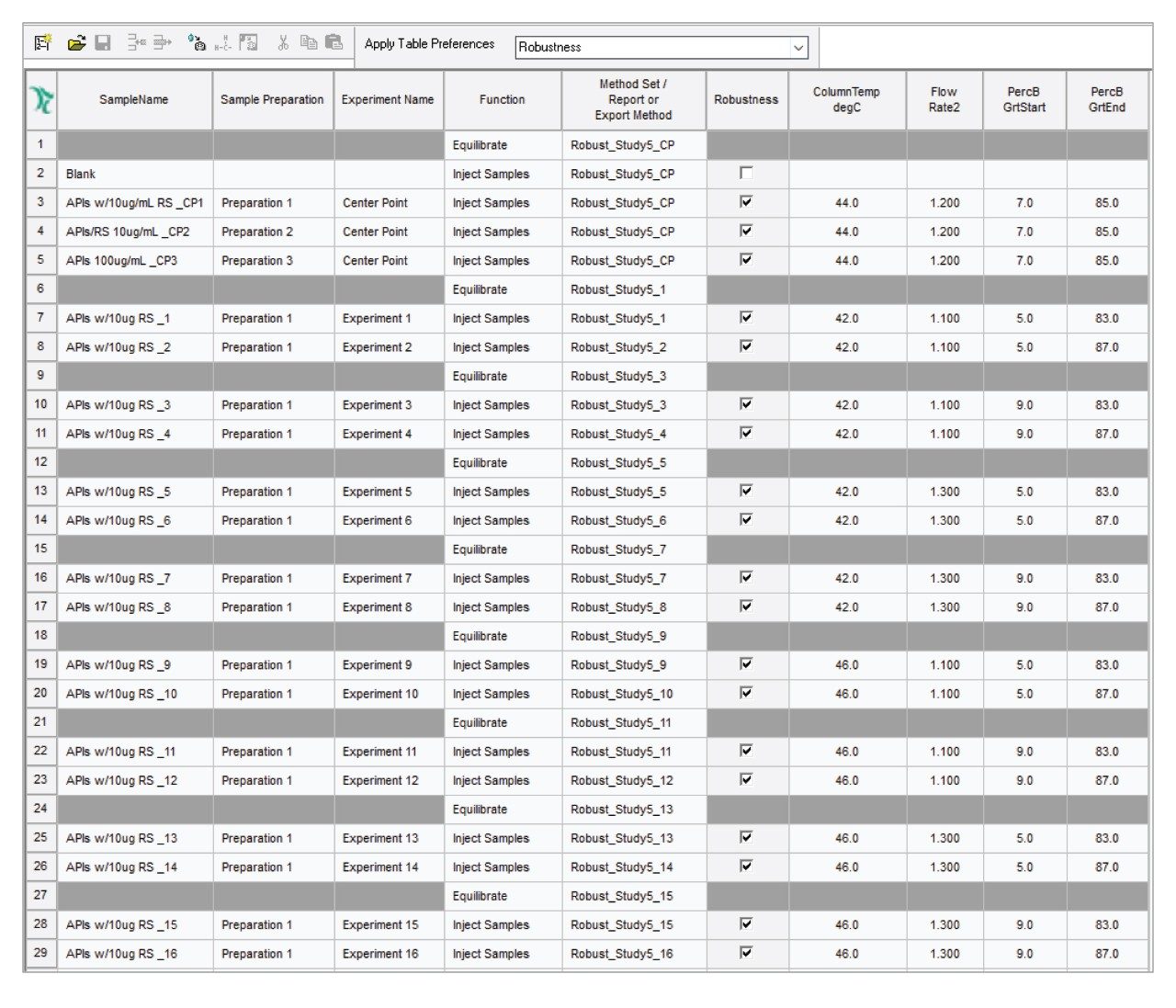
The chromatographic methods required to run the entire study were automatically created using Empower SSG Software. The Empower SSG automatically created a sample set method according to the experiment design as a ready-to-run injection sequence (Figure 3). The sample set method included the experiment name, and method sets with instruments methods for each experiment. Injections of blank and equilibration steps throughout the run were included as instructed by the user.
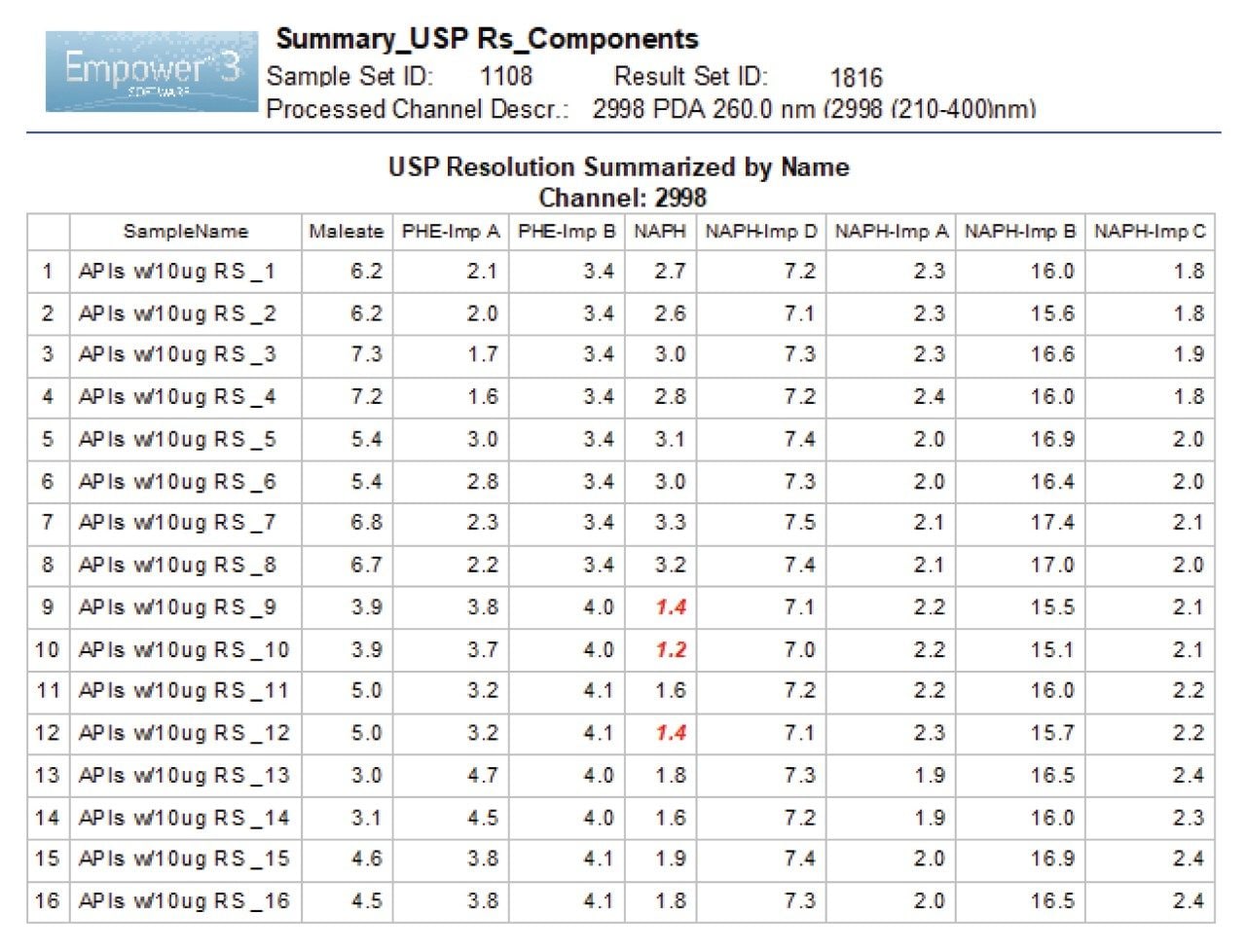
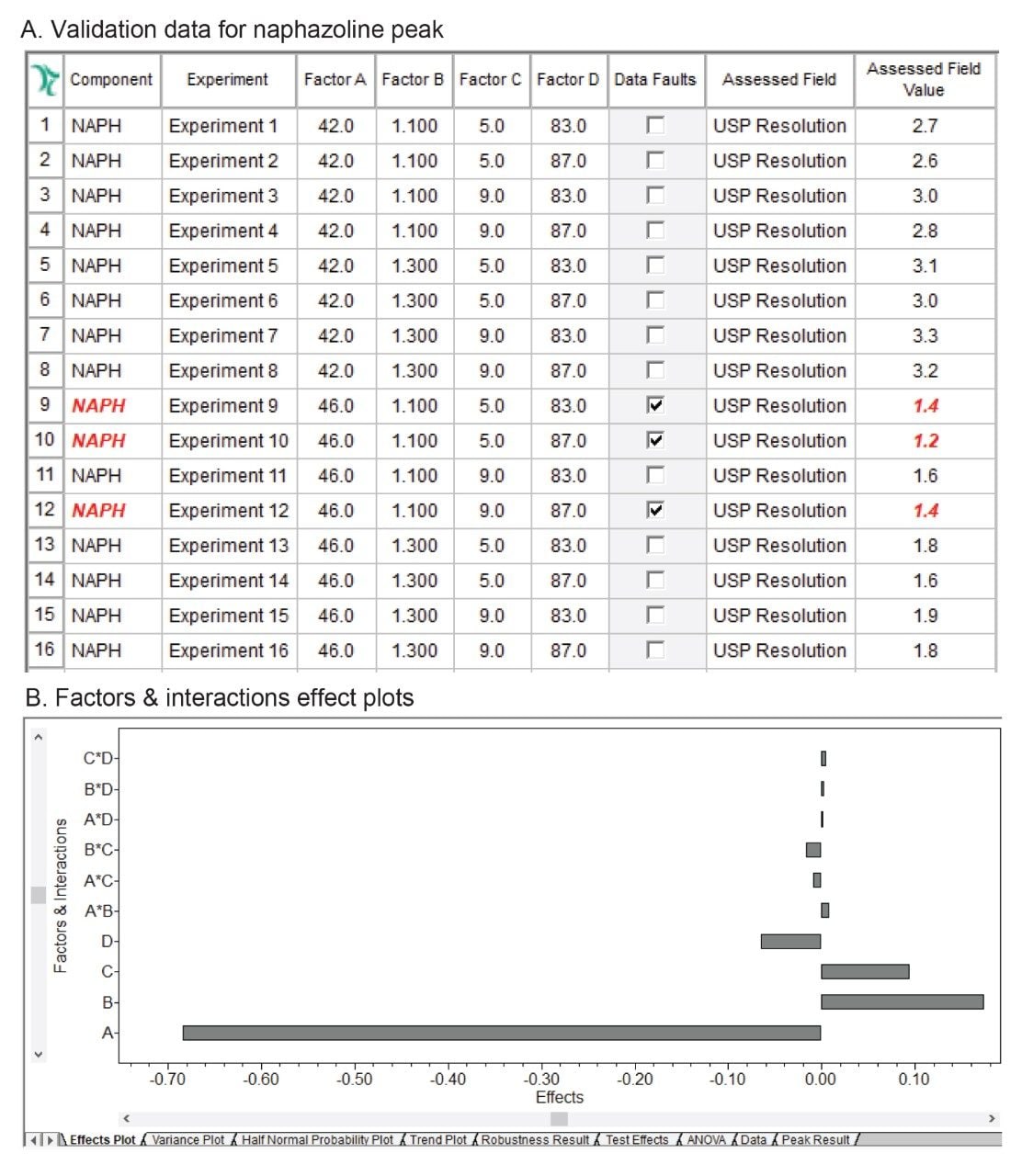
The robustness data showed that the method met the limit for a USP Resolution ≥1.5 for most compounds, except for naphazoline peak (Figure 4). The resolution for naphazoline peak in experiments 9, 10, and 12 was found to be below 1.5 and was flagged by the software. The effect plots were used to determine which factor had most the impact on the resolution by visually examining the magnitude of the effect. For NAPH API peak, factor A (column temperature) had a significant impact on the resolution with negative effect, indicating loss in resolution with increase of column temperature (Figure 5). Furthermore, factor B (flow rate) had a positive impact effect on NAPH API, improving resolution when flow rate increased. For related substances, individual factors showed positive and negative impact effects and interactions of multi-variables had minimum effects on the resolution (Figure 6).
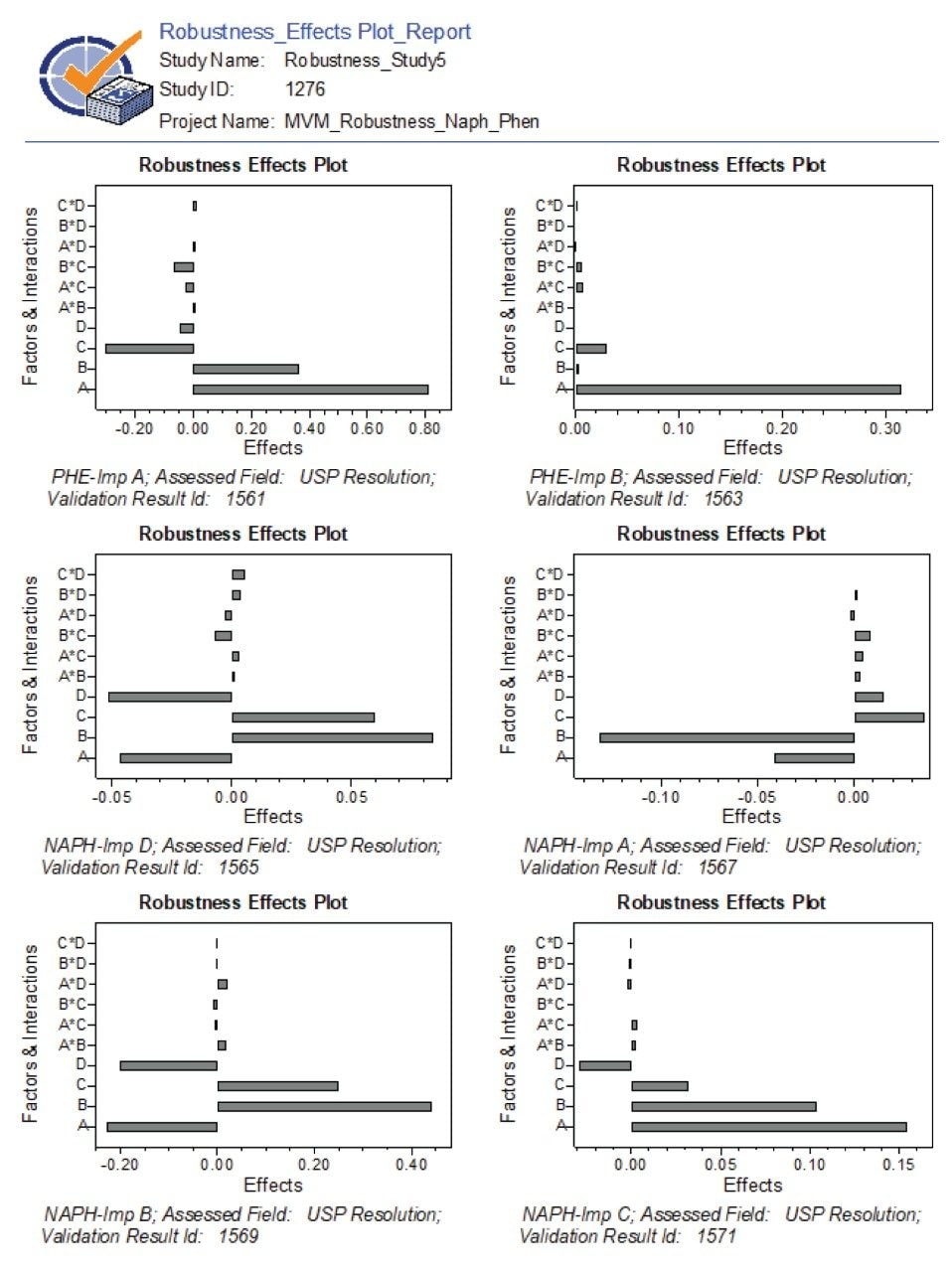
Control strategy
A control strategy consists of a set of controls to ensure that the analytical method performs as expected during routine use throughout its lifecycle and is based on the enhanced understanding of the method performance characteristics derived from risk assessment and robustness evaluation.2 Understanding the effect on the method performance facilitates identification of parameters that should be controlled and appropriately defining method’s operating range over which performance of the method is unaffected.
In this work, the DoE robustness study demonstrated sensitivity of the method to column temperature, specifically impact on the resolution between the most critical pair of peaks, PHE-Imp A and NAPH API. Restricting column temperature setting of 44.0±1.0 °C was recommended to achieve the required resolution of ≥1.5. Other parameters including flow rate of 1.2±0.1 mL/min, composition of organic at gradient start of 7.0±2.0% and gradient end of 85.0±2.0% provided acceptable operating range over which resolution criteria was met.
Conclusion
Robustness of a method for the analysis of naphazoline hydrochloride, pheniramine and associated related substances was assessed using a multivariable DoE study with Empower MVM Software. The Empower SSG Software automated generation of chromatographic methods to run the entire study. The effect plots clearly indicated parameters with the most impact on the method performance and helped to define method’s operating conditions to meet the performance criteria.
Evaluating method robustness using a multivariable DoE helps to identify the effect of method parameters and robust operating conditions. It facilitates development of a control strategy to reduce and control source of variability during routine use.
References
- U.S. FDA. Analytical Procedures and Methods Validation for Drugs and Biologics Guidance for Industry. U.S. Department of Health and Human Services, Food and Drug Administration, July 2015.
- ICH Q14. Analytical Procedure Development, International Conference on Harmonization, 31 March 2022.
- Swartz ME, Krull IS. Method Validation and Robustness. LCGC North America-05-01-2006, Volume 24, Issue 5 Published on: May 1, 2006.
- Maziarz M, Rainville P. Efficient Method Development Using Systematic Screening Protocol. Waters Application Note, 720007850 2023.
- Maziarz M, Wrona M, McCarthy SM. Increasing Efficiency of Method Validation for Metoclopramide HCl and Related Substances with Empower 3 MVM Software. Waters Application Note, 720005111, 2018.
- Empower SSG.
Featured Products
720007870, March 2023