This application note demonstrates that the ACQUITY UPLC H-Class Bio System with 2D Technology offers advantages for the on-line fractionation and desalting of challenging biological samples. A therapeutic monoclonal antibody, infliximab, was used to demonstrate this.
Characterization of charge variants in biotherapeutic proteins often relies on charge-based separation techniques such as ion exchange chromatography (IEX).1,2 While IEX separations are powerful in gathering information such as charge variant composition, the use of salts and buffer components prevents direct coupling to mass spectrometry (MS) to elucidate peak identity.3 Chromatographic fractions of interest are often manually collected and desalted offline prior to MS analysis, which negatively impacts overall analysis time and productivity.
Waters’ solution to this challenge is the ACQUITY UPLC H-Class Bio System with 2D Technology featuring a heart-cut technique. The fidelity of this 2D UPLC system makes it well suited for the automated fractionation and desalting of peaks of interest for increased productivity. Tandem column configurations (e.g., IEX/RPLC), when used with synchronized valve switching, can be used to heart-cut peaks of interest from the 1st dimension column to the 2nd dimension column. The 2nd dimension column acts as a trapping/desalting column where samples are desalted and eluted in solvents compatible with down-stream analyses, such as MS analysis or enzymatic digestion.
The objective of this three-part application series is to collectively demonstrate that the ACQUITY UPLC H-Class Bio System with 2D Technology offers advantages for the on-line fractionation and desalting (part 1) of challenging biological samples and is a viable interface for IEX with MS analysis (part 2). The system’s ability to increase productivity across platforms will also be demonstrated with the on-line enrichment of low abundance species for peptide analysis (part 3). A therapeutic monoclonal antibody, infliximab, was used to demonstrate this.
|
LC system: |
ACQUITY UPLC H-Class Bio with 2D Technology 1st dimension pump: ACQUITY UPLC Quaternary Solvent Manager, ACQUITY UPLC Column manager 2nd dimension pump: ACQUITY UPLC Binary Solvent Manager, ACQUITY UPLC Autosampler with FTN |
|
Detectors: |
(1st dimension) ACQUITY UPLC TUV (2nd dimension) ACQUITY UPLC PDA |
|
Absorption wavelength: |
280 nm |
|
Vials: |
Total recovery vial: 12 x 32 mm glass, screw neck, cap, nonslit (p/n 600000750cv) |
|
Column: |
Protein-Pak Hi Res SP, 7 μm, 4.6 x 100 mm (p/n 186004930) ACQUITY UPLC BEH C4, 300Å, 1.7 μm, 2.1 x 50 mm, (p/n 186004495) |
|
Column temp.: |
25 °C (IEX); 80 °C (C4) |
|
Sample temp.: |
4 °C |
|
Injection vol.: |
2 μL unless otherwise stated |
|
Quaternary solvent manager: |
|
|
Flow rate: |
0.500 mL/min |
|
Mobile phase A: |
100 mM MES monohydrate |
|
Mobile phase B: |
100 mM MES sodium salt |
|
Mobile phase C: |
1000 mM NaCl |
|
Mobile phase D: |
18 MΩ H2O |
|
Auto•Blend Plus setting: |
20 mM MES buffer, pH 6.5, 25–65 mM NaCl in 15 minutes |
|
Binary solvent manager: |
|
|
Flow rate: |
0.250 mL/min for heart-cut, otherwise 0.500 mL/min |
|
Mobile phase A: |
18MΩ H2O, 0.1% FA |
|
Mobile phase B: |
Acetonitrile, 0.1% FA |
|
Gradient: |
5–85% B in 10 minutes |
MassLynx Software v4.1 (SCN 8.62)
The Waters Protein-Pak Hi Res SP, 7-μm, 4.6 x 100 mm, strong cation exchange column (p/n 186004930) and ACQUITY UPLC Protein BEH C4 Column, 300Å, 1.7 μm, 2.1 x 50 mm, (p/n 186004495) were conditioned prior to use. Chemical reagents were purchased from Sigma Aldrich and used as received. The monoclonal antibody infliximab was received at a concentration of 20 mg/mL in formulation buffer.
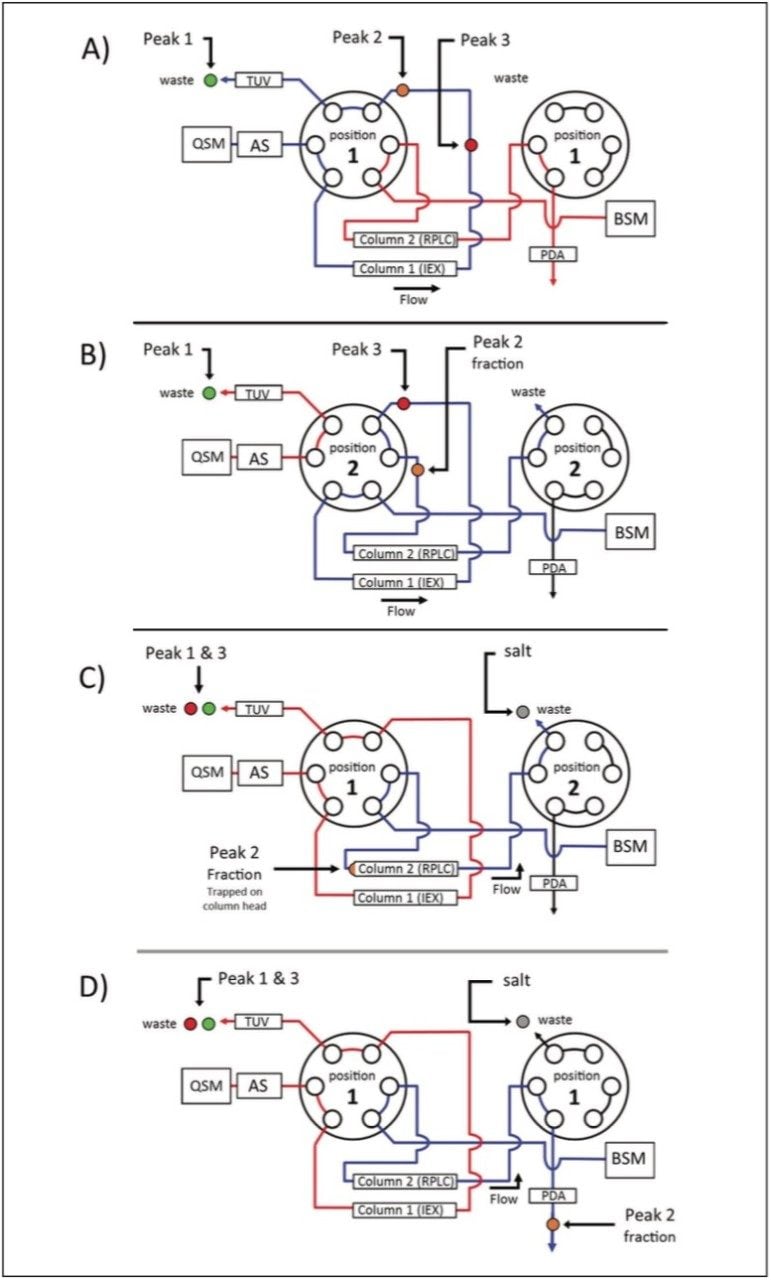
ACQUITY UPLC H-Class Bio System with 2D Technology featuring heart-cut technology The ACQUITY UPLC H-Class Bio System with 2D Technology featuring the heart-cut process is readily deployed with a two-column configuration as shown in Figure 1. With both valves in position 1 (Figure 1A), the flow from both the quaternary solvent manager and binary solvent manger are independent of each other, allowing for independent gradients to be performed on column 1 and 2.
The heart cut is performed when the valve positions are temporarily switched to position 2 (Figure 1B), combining the flow paths (Figure 1B blue trace) where effluent from column 1 is redirected to column 2. The ACQUITY UPLC Column Manager supports independent valve control as shown in Figure 1C. With the left valve in position 1 and the right valve in position 2 the flow paths of each column are isolated again, with the 2nd dimension column being eluted to waste. This allows for unbound salts to be washed from the heart-cut fraction, which is trapped at the column head of the 2nd dimension column, using the aqueous phase of the 2nd dimension.
Once desalted, the heart-cut fraction can be readily eluted in a mobile phase amenable to down-stream analyses using the ACQUITY UPLC Binary Solvent Manager.
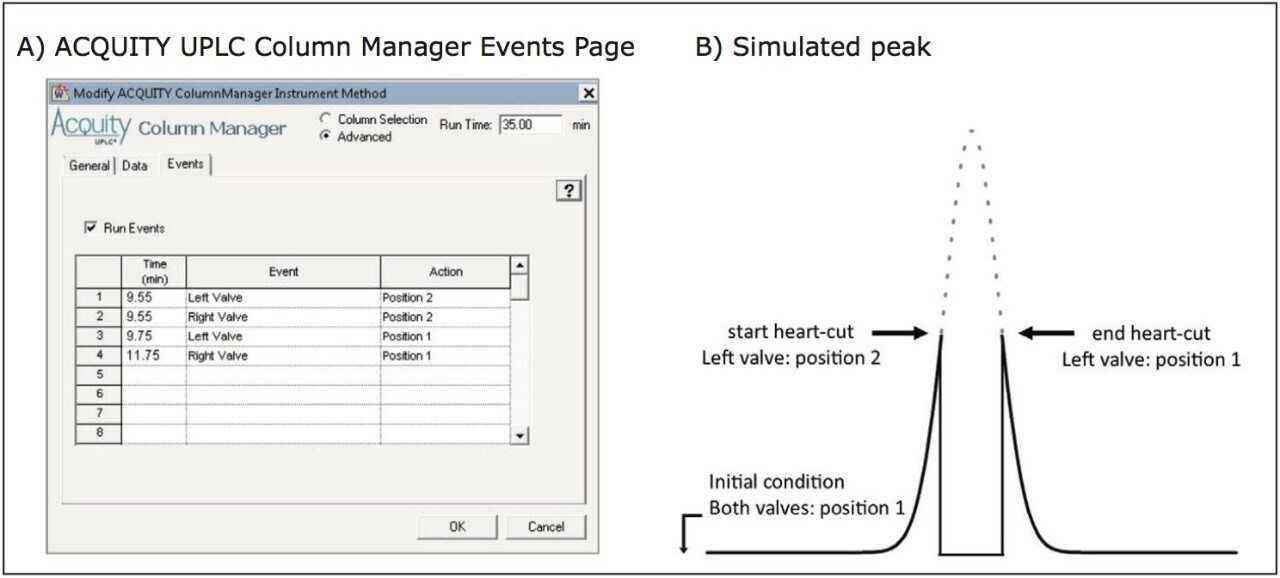
The heart-cut process is readily engaged using the integrated event table of the ACQUITY UPLC Column Manager as shown in Figure 2A. When enabled the event table allows for independent programming of the left and right valve and their respective position. An illustration of the expected effect of the valve switches on the chromatographic profile are shown in Figure 2B where the “heart” of the peak is re-directed to the 2nd dimension column, bypassing the ACQUITY UPLC TUV Detector as illustrated in Figure 1B, resulting in the characteristic profile. Adaptable column configurations, combined with an easy-to-use integrated interface, demonstrates the ACQUITY UPLC H-Class Bio System with 2D Technology is well suited for fractionation, desalting of challenging biological samples.
Biotherapeutics are often processed in non-denaturing buffers containing salts, surfactants, and sugars. Additives such as these often need to be removed via desalting/buffer exchange columns or diluted to facilitate down-stream analyses that incorporate mass spectrometry or enzymatic digests. The heart-cut technology featured in the ACQUITY UPLC H-Class Bio System with 2D Technology is readily deployed with a two column configuration (IEX/RPLC) as shown in Figure 1 for on-line desalting of biotherapeutics.
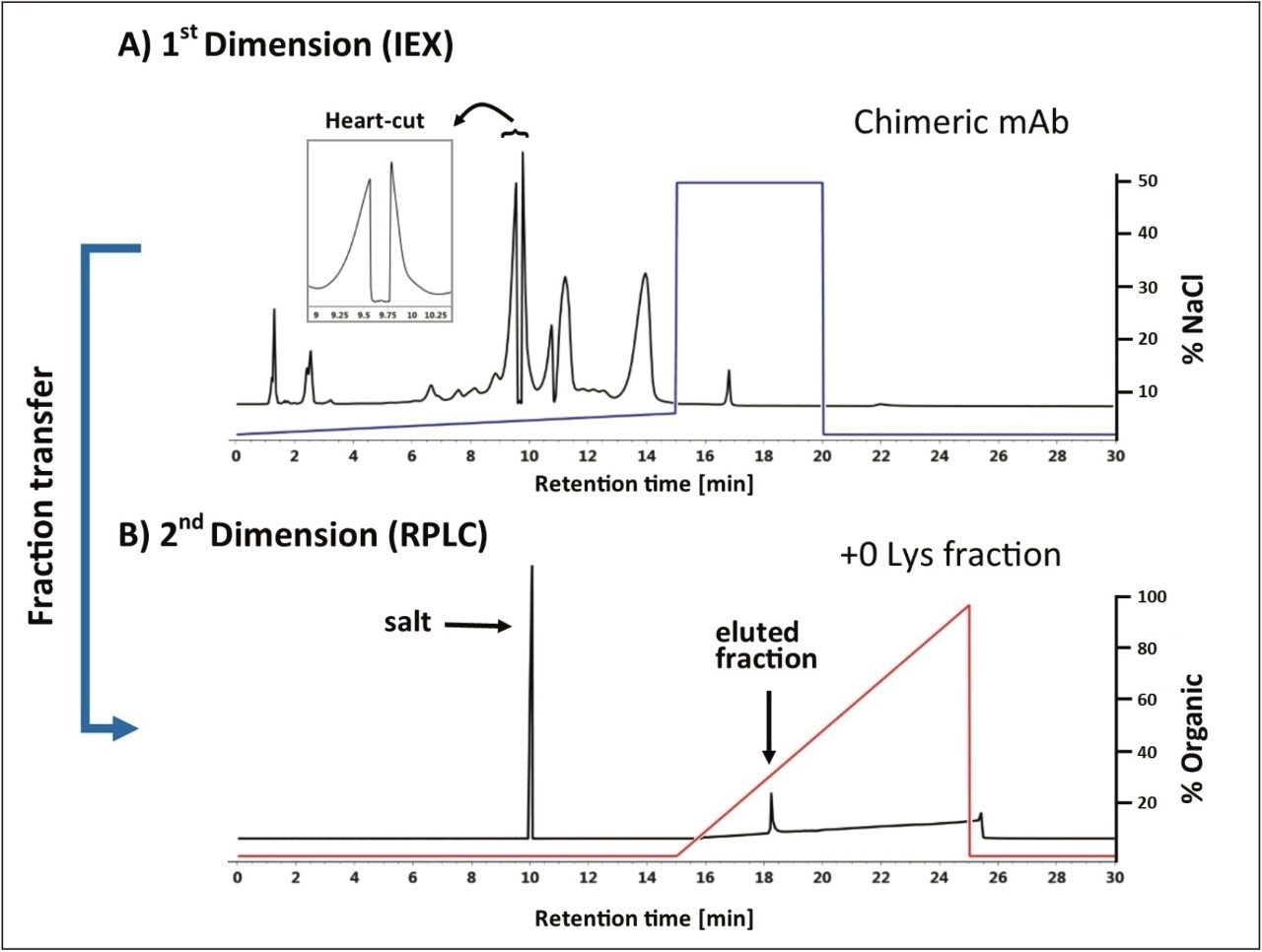
The Waters Protein-Pak Hi Res SP, 7 μm, 4.6 x 100 mm, SCX Column was used as the 1st dimension column with the Auto•Blend Plus Technology delivering a 15-minute salt gradient from 25–65 mM NaCl in 20 mM MES buffer, pH 6.8. Figure 3 shows a deglycosylated sample of infliximab where a heart-cut is being performed on the +0 lysine truncation variant from 9.55 min to 9.75 min using the ACQUITY UPLC Binary Solvent Manager Pump at a flow rate of 0.250 mL/min to elute the fraction to the 2nd dimension column.
As seen in the inset of Figure 3A the optical trace when performing the heart-cut exhibited the characteristic profile as illustrated in Figure 2B. The eluent flow containing the fraction of interest was redirected to an ACQUITY UPLC BEH300 C4 Column (2nd dimension) where it was retained at the head of the column. The heartcut fraction was desalted for 5 minutes at a flow rate of 0.500 mL/min using 100% of the aqueous component of the 2nd dimension column (H2O, 0.1%FA v/v) as shown in Figure 3B. The desalted fraction of interest was eluted in the 2nd dimension using a 10 minute gradient from 0%–95% acetonitrile 0.1% FA, starting at the 15 minute mark using the ACQUITY UPLC Binary Solvent Manager.
The successful fractionation and desalting of a heart-cut fraction demonstrates the utility of using an ACQUITY UPLC H-Class Bio System with 2D Technology to increase efficiency in sample preparation of complex biotherapeutics.
Increasing demand for informational content in the characterization of biotherapeutics, combined with a fast-paced work environment, require efficient solutions that offer flexibility. The ACQUITY UPLC H-Class Bio System with 2D Technology is Waters’ solution to these challenges. The heart-cut feature offered with the system is well suited for fractionation and desalting of challenging biological samples. Compatibility with multiple column configurations and the ability to automate the process offers today’s analyst the flexibility needed to maximize information in the characterization of biotherapeutics without compromising productivity.
720005329, March 2015