This application brief demonstrates the benefits of using difluoroacetic acid as an alternative ion pairing agent for improved optical performance and ionization efficiency in dual detector, LC-UV/MS workflows.
Use of difluoroacetic acid as an ion-pairing agent improves optical peak shape and increases MS response for peptide LC-UV/MS analyses in comparison to formic acid or trifluoroacetic acid.
Mobile phase additives such as formic acid (FA) and trifluoroacetic acid (TFA) are commonly used in reversed phase liquid chromatography (RPLC) to improve analyte retentivity and peak shape. For biopharmaceuticals, the quality of data and effective separation of peptide mixtures or complex large proteins can often be improved through the combined use of optical and mass spectrometry (MS) data in analytical LC-UV/MS workflows. However, FA and TFA do not necessarily facilitate dual detector workflows. Being a weaker ion pairing agent, FA produces favorable MS intensity compared to TFA, which causes significant MS signal suppression. However, FA, compared to TFA, generates noisier baselines and broader peaks in UV-based assays. As LC-UV/MS-based workflows become more ubiquitous for peptide analysis, the need for an ion pairing reagent that produces more ideal UV chromatograms, as well as minimal MS signal suppression, becomes increasingly important, particularly for the development of efficient methods for assaying the quality attributes of biotherapeutics.
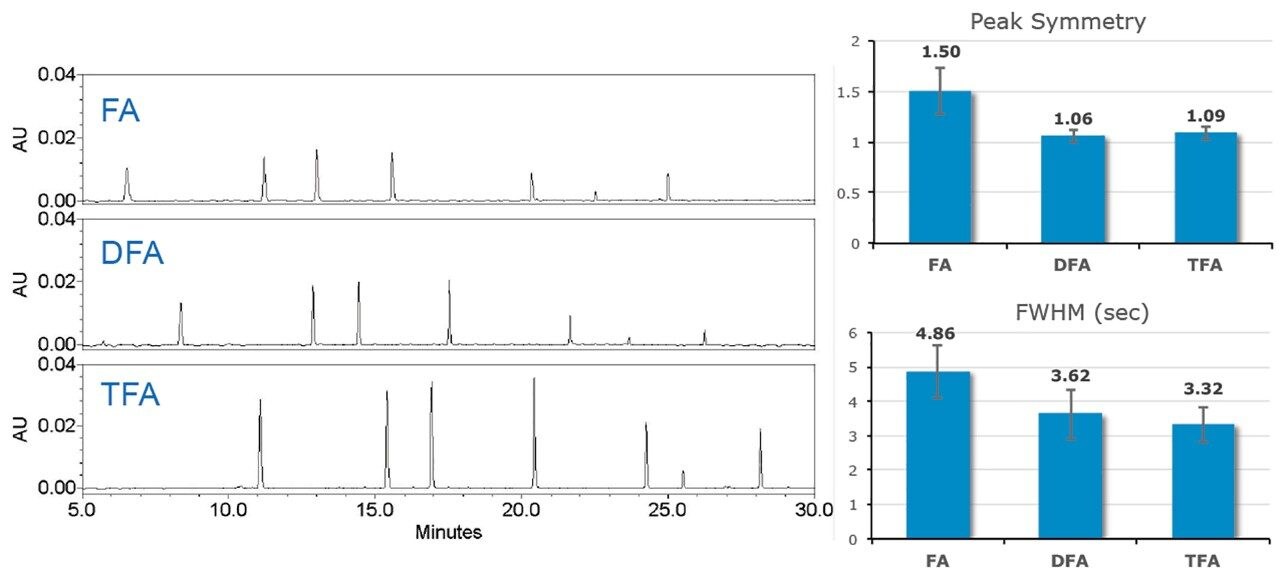
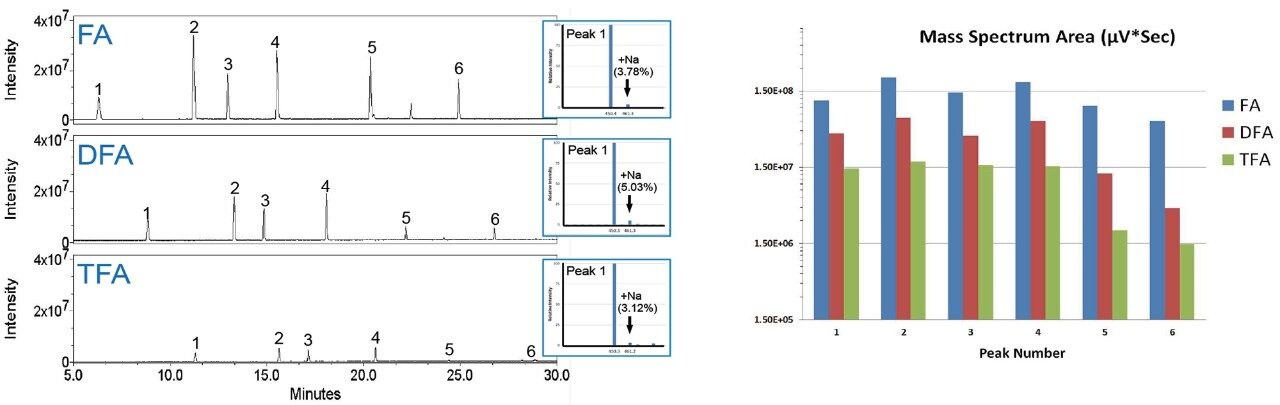
In addition to having effects on ionization efficiency, an ion pairing reagent can have considerable effects on retentivity, separation efficiency, chromatographic resolution, and chromatogram baseline properties. For LC-based separations, an ideal peak is defined as being Gaussian with a tailing factor of 1.0 and a narrow peak width. To this end, MS-grade difluoroacetic acid (Waters IonHance Difluoroacetic Acid) was evaluated against formic acid and trifluoroacetic acid as a novel ion pairing agent for peptide LC-UV/MS. Six peptides from the Waters MassPREP Peptide Mixture were analyzed under RPLC conditions on an ACQUITY UPLC H-Class Bio System configured with an ACQUITY QDa Mass Detector in-line with optical detection (ACQUITY TUV). Mobile phases of water and acetonitrile were prepared with 0.1% (v/v) FA, DFA, or TFA. As shown with the UV chromatograms in Figure 1, DFA falls midway between FA and TFA in terms of ion pairing strength and affording a change in retentivity. Furthermore, DFA resulted in an average improvement of 25% to peak width and an 82% improvement in peak symmetry in comparison to FA. These are improvements that are comparable to those seen with TFA, which were 30% and 84%, respectively. Similarly, DFA was observed to yield an MS-response (Figure 2) between that of FA and TFA. Compared directly to TFA, DFA produced an approximately three-fold increase in detector response. In addition, the spectral quality of the IonHance DFA was found to be comparable to that of the employed, MS-grade FA and TFA reagents. As noted in the insets of Figure 2, salt adducts of Na+ were at or below 5% across the peptides evaluated. The observed improvement in chromatographic performance and increased spectral response makes IonHance DFA an ideal ion pairing agent for LC-UV/MS workflows that require a balance between UV and MS performance.
Selection of ion pairing conditions to produce both quality optical and mass spectrometric data in RPLC-based LC-UV/MS workflows has been historically challenging. Formic acid is favored when MS data is prioritized and trifluoroacetic acid is preferred when optimal optical response is needed. This work demonstrates the benefits of using difluoroacetic acid as an alternative ion pairing agent for improved optical performance and ionization efficiency in dual detector, LC-UV/MS workflows.
720006482, January 2019