This is an Application Brief and does not contain a detailed Experimental section.
The goal of this study is to maximize the utility of ion-exchange mass spectrometry (IEX-MS), mobile phases containing ammonium bicarbonate or carbonate should be avoided.
Native MS-based proteomics should avoid the use of either ammonium bicarbonate or carbonate.
Direct hyphenation of ion-exchange chromatography (IEX) to mass spectrometry (MS) is a relatively new advancement that has allowed for the online characterization of native proteins, such as biotherapeutic monoclonal antibodies. Three distinct types of gradients have been utilized to date: salt,1–2 pH,3 and salt-mediated pH.4 Salt-mediated pH gradients offer the broadest utility as they use an increase of pH with a concurrent ionic strength gradient.
Published examples of IEX MS have used various types of mobile phases with the majority using ammonium-based solutions. Counter ions have included formate, acetate, and bicarbonate.
Ammonium formate, as a choice for native protein IEX-MS analysis is less than ideal because of its disparate pKas, which leaves a relatively large unbuffered region around neutral pH values.5 The unbuffered region leads to unoptimized separations and irreproducible elution. The use of ammonium bicarbonate poses a different challenge. While its pKas lend themselves to good buffering capacity near neutral and basic pH values, ammonium bicarbonate can cause significant problems during electrospray ionization due to the in situ formation of CO2. With this comes the problematic formation of CO2 adducts, which needlessly complicates MS data. Additionally, protein denaturation is observed when using bicarbonate, bringing into question whether a native state analysis is even possible with such mobile phase components.6–8 Finally, proteins become supercharged to higher charge states and are shifted into an inherently noisier m/z window and all of these effects are observed even at low ionic strengths (e.g., 5 mM ammonium bicarbonate).
Experiments on mobile phase effects have been performed by doping IonHance CX-MS Buffers with various quantities of ammonium carbonate in a manner that did not significantly change their pH (≤0.3 pH shifts). Carbonate salt, rather than ammonium bicarbonate, was utilized for these experiments because it was more readily available in MS-grade purity. Despite this substitution, it can be assumed that the primary form of the ammonium counterion is bicarbonate, as it was added into a pH controlled mobile phase.
From this work, we have come to realize that the use of bicarbonate eliminates any correspondence between UV and mass spectral data. Our data suggest a disconnect, originating at the ESI source, that effectively splits the UV chromatogram from the MS chromatogram. In turn, peak identification is made significantly more difficult. In contrast, IEX-MS with ammonium acetate mobile phases shows reproducible continuity between UV and MS chromatograms.
The solution is simple: the use of bicarbonate- and carbonate-based mobile phases should be avoided when looking to perform native protein LC-MS. Figure 1 illustrates the identification challenges inherent to (bi)carbonatebased analyses; the introduction of spurious peaks in the TIC compared to the UV chromatogram reduce confidence in peak identification. The addition of peaks to the TIC is suggestive of a reaction taking place in the source. Furthermore, the introduction of a mere 5 mM concentration of ammonium carbonate is sufficient for the generation of these peaks. Additionally, supercharging and the corresponding appearance of more highly charged ions are observed at both the intact and subunit level (Figures 1 and 2). Previously published work with myoglobin revealed some of the additional charge state series were denatured forms of the protein.6 In the confined space of an ESI droplet, supercharging can lead to partial unfolding and denaturation of a protein.7-8
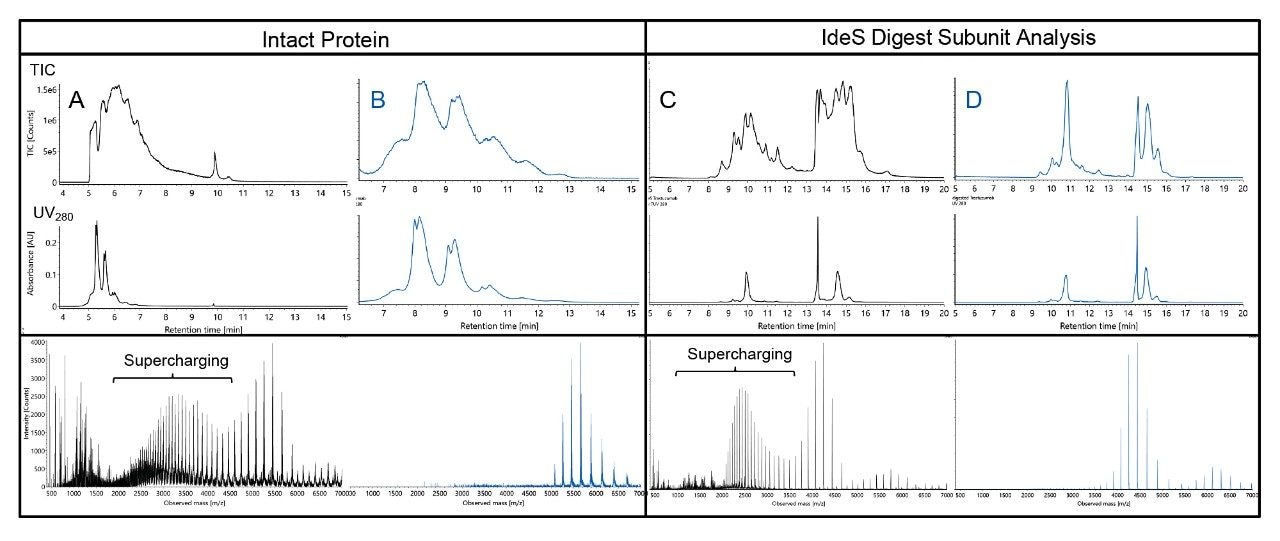
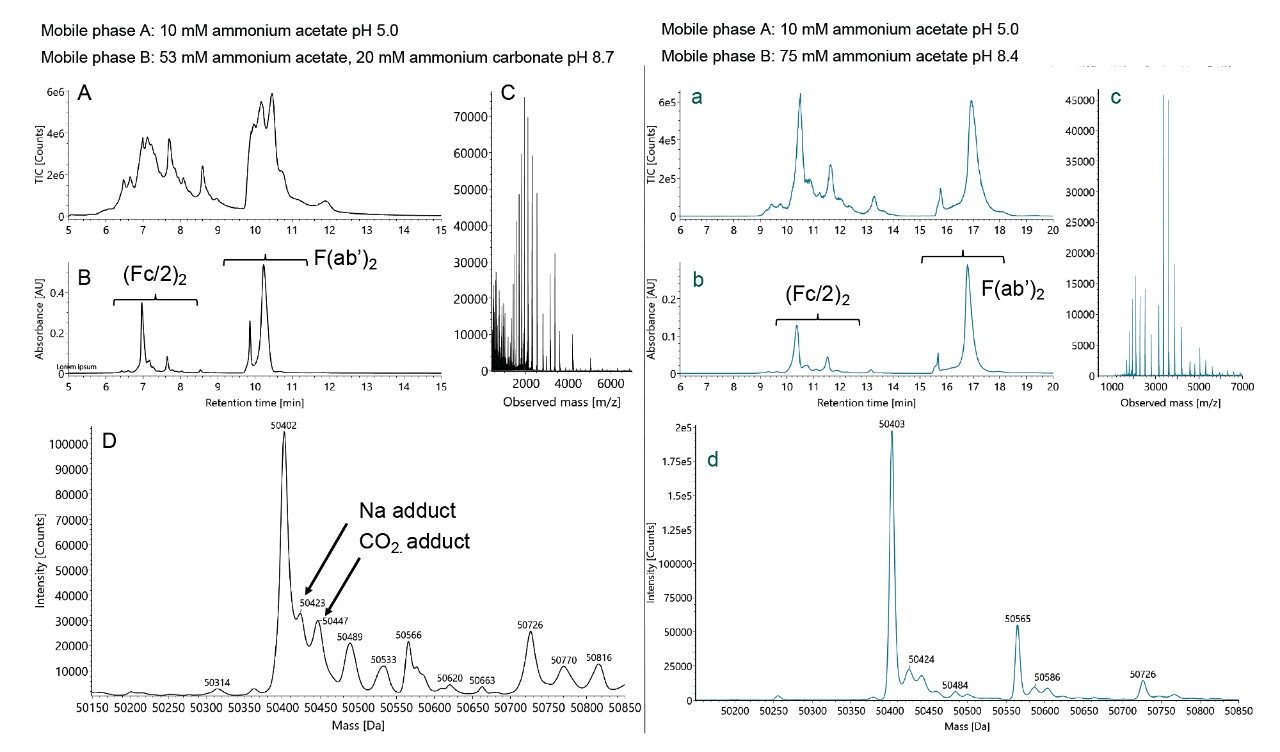
Increasing the concentration of ammonium carbonate in mobile phase B exacerbates each of these features, and molecular weight calculations reveal the presence of +44 Da adducts, even for an (Fc/2)2 subunit that is eluted with a small percent composition of (bi)carbonate containing eluent (Figure 2). Upon working through example data analysis, it was also observed that the (bi)carbonate methods yielded comparatively broader deconvoluted peaks, higher adduct levels, and a reduced ability to identify low abundance proteoforms, such as the glycoforms in a mAb sample.
To maximize the capabilities of IEX-MS, an analyst must carefully consider mobile phase composition. The use of bicarbonate and carbonate are contraindicated due to mismatched TIC and UV chromatograms, protein supercharging and denaturation, and formation of CO2 adducts. In contrast, IonHance CX-MS pH Concentrates, and their corresponding ammonium acetate mobile phase compositions, have been carefully formulated to deliver robust, reproducible methods and to facilitate straightforward peak identification.
720006737, January 2020