
Complete residue analysis of steviol glycosides in food, is a challenge due to nature of the task, i.e., the detection of low steviol glycoside isomer concentrations in complex food commodities, where generic extraction procedures have been used. The challenge is two-fold where low concentrations are present, because they may be used in conjunction with other sweeteners or sugars.
In addition, detection at low concentrations is difficult where one or two of the glycosides are at very high concentrations (e.g. rebaudioside A). Isomers of substances may have different chemical properties – they can have different flavor, as well as possible variability in their absorption, distribution, metabolism, elimination, and toxicity.
Hence it is necessary to have information on the make up of substances that can contribute to flavor.
An initial highly selective, sensitive screening method could be used, where the focus is only aimed towards qualitative, but much more specific detection is required to determine the sweetener’s purity, as the purity can impact taste. In this application note, we present a unique approach to screen food products for steviol glycosides using ionKey/MS and ion mobility mass spectrometry (IM-MS), which provides unequivocal specificity and sensitivity.
Stevia rebaudiana Bertoni is a perennial shrub of the Asteraceae (Compositae) family native to regions of South America. Stevia is of significant economic value due to the high content of natural, dietetically valuable sweeteners in its leaves. It is referred to as “the sweet herb of Paraguay”. Currently, the stevia plant or its extracts are used as sweeteners in North America, South America, Asia, and some European countries. The Joint FAO/WHO Expert Committee on Food Additives (JECFA) has established regulations for steviol glycosides demanding a purity level of at least 95% of the seven chemically defined steviol glycosides.1
Complete residue analysis of steviol glycosides in food, is a challenge due to nature of the task, i.e. the detection of low steviol glycoside isomer concentrations in complex food commodities, where generic extraction procedures have been used. The challenge is two-fold where low concentrations are present, because they may be used in conjunction with other sweeteners or sugars. In addition, detection at low concentrations is difficult where one or two of the glycosides are at very high concentrations (e.g. rebaudioside A). Isomers of substances may have different chemical properties – they can have different flavor, as well as possible variability in their absorption, distribution, metabolism, elimination, and toxicity. Hence it is necessary to have information on the make up of substances that can contribute to flavor. An initial highly selective, sensitive screening method could be used, where the focus is only aimed towards qualitative, but much more specific detection is required to determine the sweetener’s purity, as the purity can impact taste.2-4
Full scan high resolution mass spectrometry (HRMS) offers high specificity with theoretically no limitation in the number of compounds that can be detected. The continued technology advances of time-of-flight (Tof) mass spectrometry have brought higher sensitivities, resolution, and mass accuracy (typically sub-2 ppm). Tof MS is used in combination with time tolerances, isotopic matching, fragment ions/ratios, and response thresholds to help reduce false positive and false negative detections in screening assays.
Advances in mass spectrometry have vastly improved sensitivity for full spectral analysis, but further enhancements would improve the mass spectral data quality. This is especially important to avoid compromised precursor ion or fragment ion information, and ensure high mass accuracy at low levels. Despite these MS enhancements it can still be a challenge to rapidly and efficiently identify targeted isomeric compounds present in a sample, particularly with large numbers of co-extracted matrix components. Improvements in sensitivity, and the other benefits of using the ionKey/MS System have been described previously,5 including enabling sample dilution to reduce matrix suppression, and subsequently increasing the overall analyte signal-to-noise values that can be achieved.
In this application note, we illustrate the selectivity of collision cross section (CCS) measurements used in combination with other recent MS technology enhancements for profiling complex mixtures. A combination of high resolution mass spectrometry and high efficiency ion mobility based measurements and separations is used. Ion mobility is a rapid orthogonal gas separation phase technique that allows another dimension of separation to be obtained within an LC timeframe. Compounds can be differentiated based on size, shape, and charge.
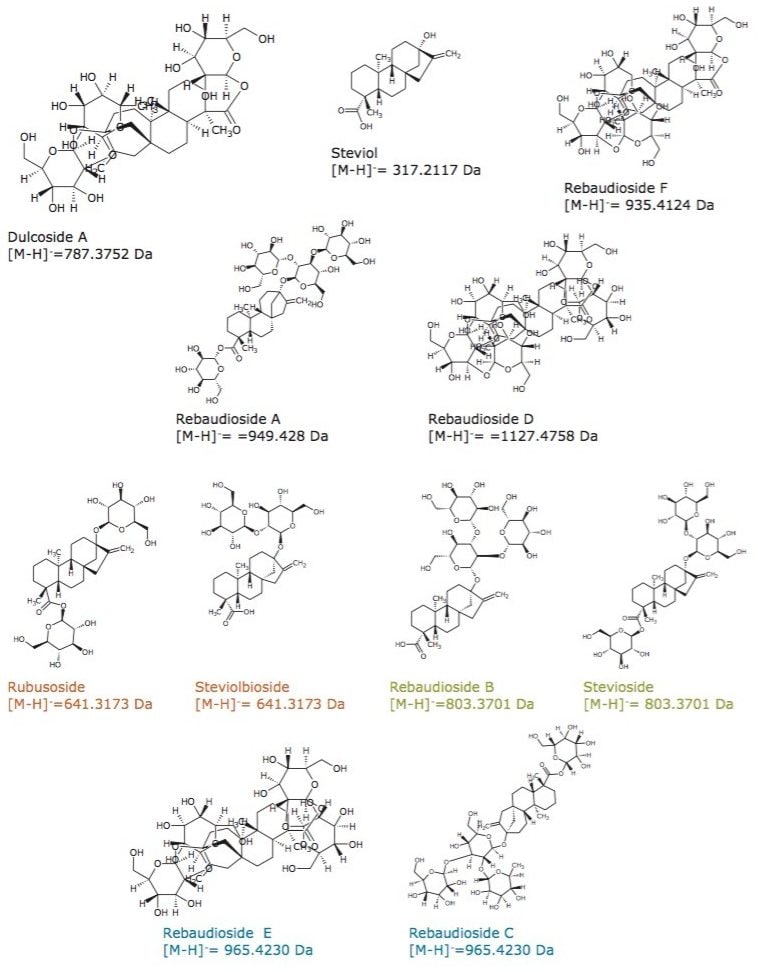
A CCS value is a robust and precise physicochemical property of an ion. It is an important distinguishing characteristic that is related to its chemical structure and three-dimensional conformation.6 Using nitrogen-based travelling wave collision cross sections (TWCCSN2) measurements can increase non targeted screening specificity. Previously generated TWCCSN2 measurements have been entered into the UNIFI Scientific Library. This allows the expected and determined TWCCSN2 values to be utilized for screening and confirming the presence of steviol glycosides. Here we present a unique approach to screen food products for steviol glycosides using the ionKey/MS System and ion mobility mass spectrometry (IM-MS), which provides unequivocal specificity and sensitivity. The structures of steviol and the steviol glycosides of interest are shown in Figure 1.
|
LC system: |
ACQUITY UPLC M-Class System |
|
Mobile phase A: |
100% Water (0.1% formic acid) |
|
Mobile phase B: |
100% Acetonitrile (0.1% formic acid) |
|
Flow rate: |
2.0 μL/min |
|
Injection volume: |
1 μL (full loop) |
|
Separation device: |
iKey BEH C18 PCA Separation Device, 130Å, 1.7 μm, 150 μm x 50 mm |
|
Separation device temp.: |
40 °C |
|
Time (min) |
Flow rate (mL/min) |
%A |
%B |
|---|---|---|---|
|
0.0 |
2 |
99 |
1 |
|
1.0 |
2 |
99 |
1 |
|
3.0 |
2 |
90 |
10 |
|
5.0 |
2 |
70 |
30 |
|
13.0 |
2 |
1 |
99 |
|
15.0 |
2 |
1 |
99 |
|
15.1 |
2 |
99 |
1 |
|
17.0 |
2 |
99 |
1 |
|
MS system: |
SYNAPT G2-Si |
|
Ionization mode: |
ESI- |
|
Capillary voltage: |
2.6 kV |
|
Sample cone voltage: |
20 V |
|
Lock mass and CCS: |
Leucine enkephalin, [M-H]- -554.2620 |
|
Acquisition range: |
50 to 1,200 m/z |
|
Acquisition rate: |
10 spectra/sec |
|
Collision energy ramp: |
30 to 70 eV |
|
Resolution: |
20,000 FWHM (Res mode) |
|
IMS T-Wave velocity ramp: |
Start: 1,000 m/s End: 300 m/s |
|
IMS T-Wave pulse height: |
40 V |
|
IMS gas flow: |
90 mL |
|
IMS duty cycle: |
10.8 ms |
Blank chocolate spread sample was purified in two steps. First, fat removal was performed by liquid-liquid extraction. The fat-free extract was subjected to a C18 solid phase extraction to remove other matrix components. The final extract was dissolved in acetonitrile. At the end of the sample preparation step, the matrix content in the extract was 0.1 g/mL.
10 steviol glycosides + steviol at ≤1 mg/mL in methanol (Table 1) were used to prepare dilutions for matrix fortification post cleanup.
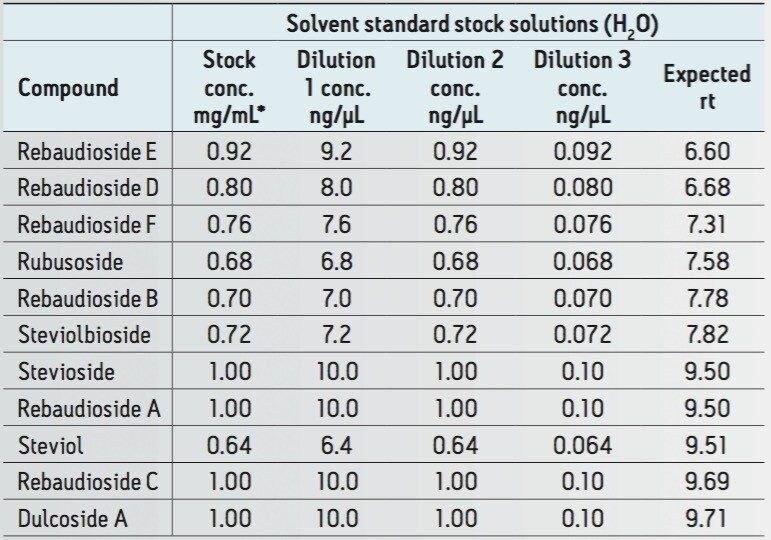
Table 1. Concentration of solvents standards used to prepare stevioside spiked chocolate spread extract samples.
* Indicated initial concentrations are sub 1 mg/mL for 7 out of 11 analytes used.
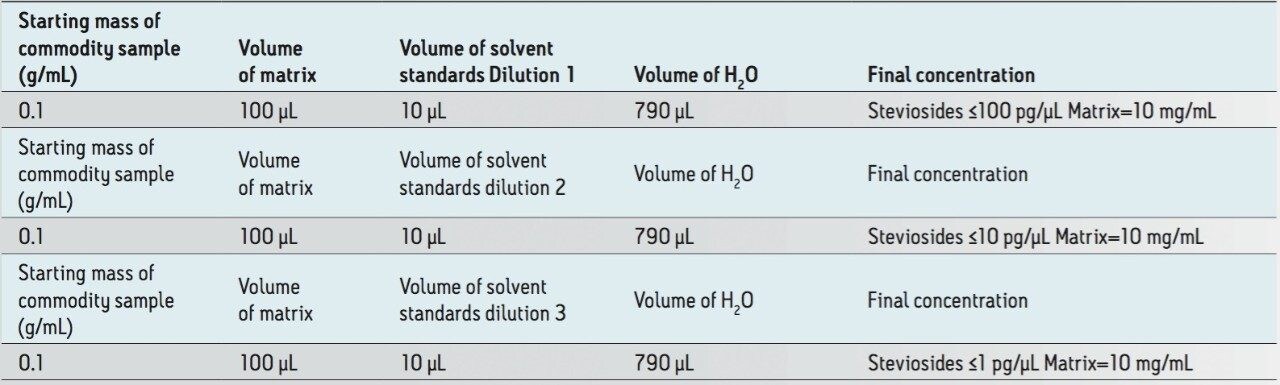
Fortification was performed on blank processed matrix after cleanup to avoid any recovery problems (Table 2). The strategy undertaken in conjunction with CCS profiling, was to determine detection levels in a complex matrix, hence acquisitions were performed with a constant matrix concentration of 10 mg/mL (Table 3).
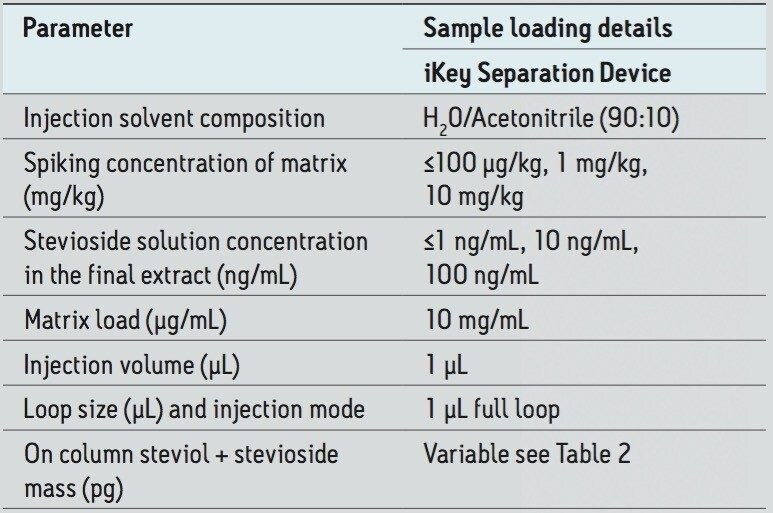
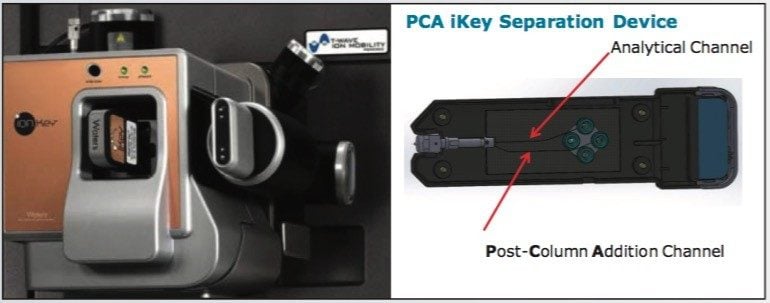
A Post Column Addition iKey PCA Separation Device (p/n: 186007580), shown in Figure 2, incorporates a 1.7 μm, ACQUITY UPLC BEH C18, stationary phase in a 150 μm diameter separation channel. The iKey Separation Device temperature was set to 40 °C, and the eluent from the separation channel flows directly to an integrated ESI emitter. All microfluidic, gas, and electrical connections are automatically engaged when the iKey Separation Device is inserted into the source enclosure and locked into place. The device incorporated an additional channel, enabling post column addition of IPA solvent. The make up solvent was configured to be delivered from channel A of the MS system fluidics for this feasibility study.
In this feasibility study, unique sensitivity and selectivity for screening steviol glycosides in complex mixtures has been achieved. Nitrogen based travelling wave collision cross sections (TWCCSN2), accurate mass, fragment ions, and retention time have been obtained to profile the steviol glycosides rebaudioside A to F, rubusodside, steviol, dulcoside A, steviolbioside, and stevioside. CCS measurements were readily obtained for the marker standards at ≤100 pg/μL, and this information was used to create a scientific library within UNIFI incorporating the expected steviol glycoside TWCCSN2 values. Previous studies have shown the benefits of TWCCSN2 screening, including spectral cleanup, avoidance of false positives, and discovery of pesticide protomers.7-9 The challenge of the assay is further complicated by the requirement to unequivocally identify three pairs of isomers. The steviol glycosides readily fragment and can be prone to insource fragmentation, resulting in isomeric fragments that can result in false positive identifications.10
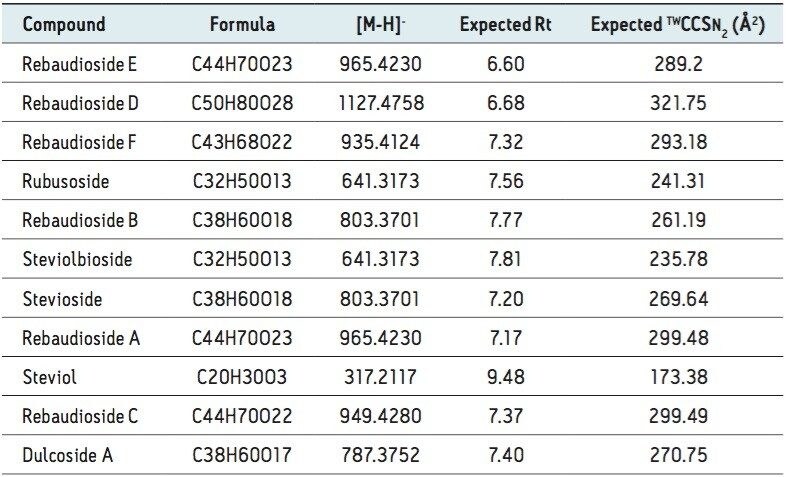
A chocolate spread extract (10 mg/mL) was spiked with the analytes and analyzed using the ionKey/MS System combined with ion mobility, then screened against the stevioside TWCCSN2 library within UNIFI. The TWCCSN2 assignment for glycosides isomer pairs (rubusodside 241.31 Å2/steviolbioside 235.78 Å2), (rebaudioside B 261.19 Å2/stevioside 269.64 Å2), and (rebaudioside A 298.9 Å2/rebaudioside E 289.2 Å2), shown in Table 4, have been determined using stevioside standards. For steviol and the remaining steviosides, CCS measurements were also determined: steviol (173.38 Å2), dulcoside A (270.75 Å2), rebaudioside F (293.18 Å2), rebaudioside C (299.49 Å2), and rebaudioside D (321.75 Å2).
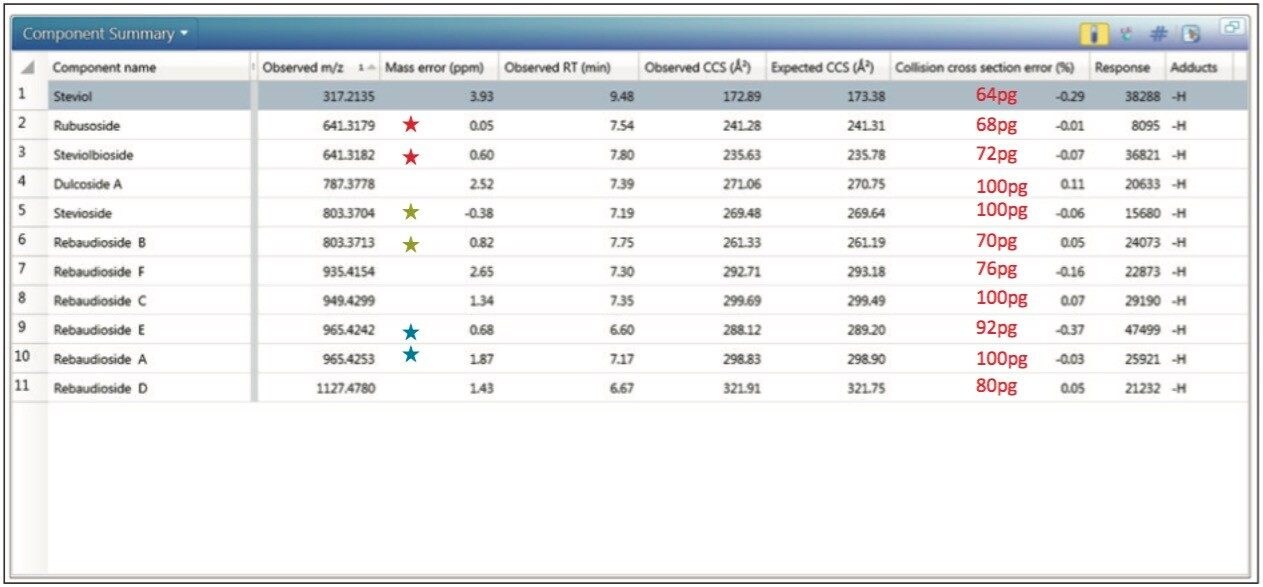
The expected TWCCSN2 and measured TWCCSN2 values for steviosides spiked into chocolate spread extract are presented in Table 4. The UNIFI Component Summary results obtained (Figure 3) clearly show the benefits of using CCS measurements and the ionKey/MS System with ion mobility. When comparing the expected and measured collision cross sections, the TWCCSN2 measurement errors were typically <0.4%, and the mass measurement error RMS=1.85 ppm obtained for ≤100 pg on column loading (actual concentrations have been added (Figure 3 in red text).
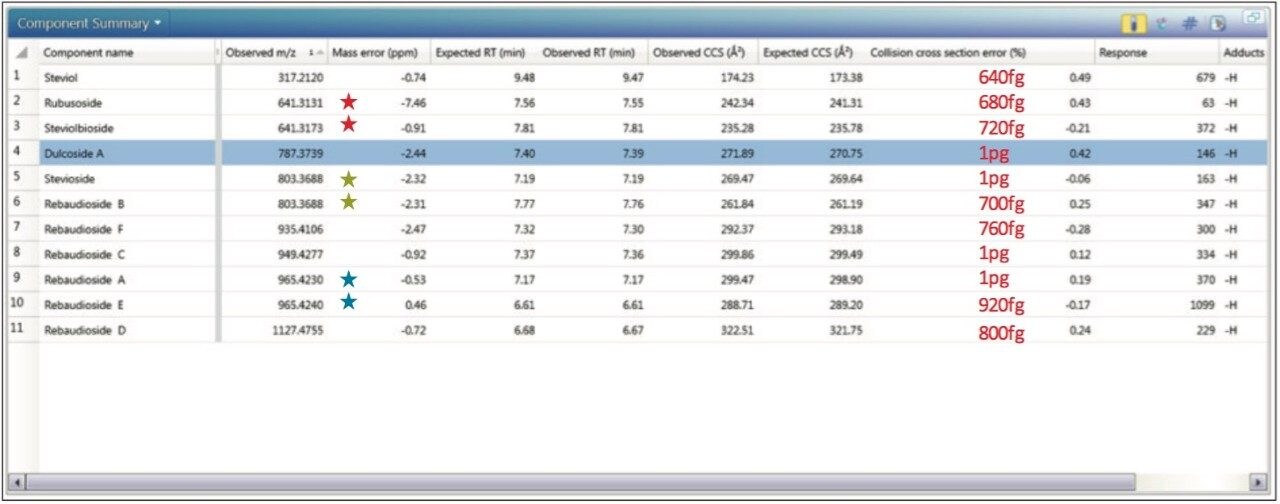
In Figure 4, the results obtained at ≤1 pg/μL are presented in the UNIFI Component Summary, where an overall RMS error of 2.72 ppm was obtained. However the mass error for rubusoside, was -7.46 ppm, where the peak area response was just 63. At this low level the mass measurement error has been affected by other more abundant ions from the matrix background. Screening with a mass meaurement tolerance of 10 ppm and CCS% tolerance of 2% has enabled rubusoside to be identified with confidence at trace levels (680 fg) and false negative detections have been avoided.
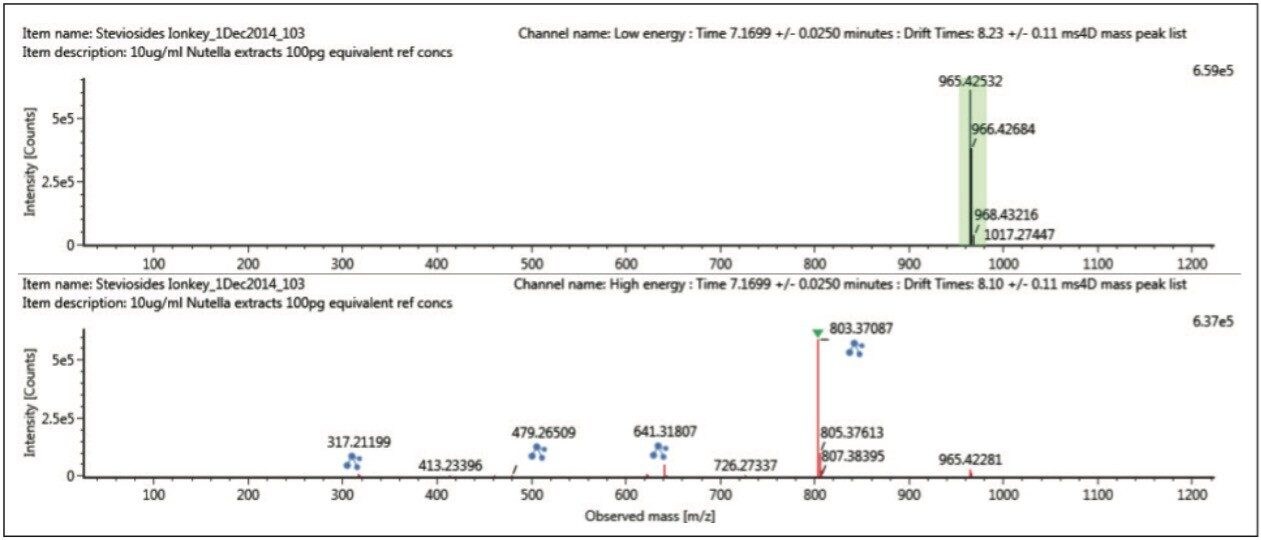
The combined extracted mass chromatogram for steviol and profiled steviosides ≤1 pg/μL spiked into chocolate spread extract are shown in Figure 5. It can be seen that stevioside and rebaudioside A coelute at 7.18 minutes.
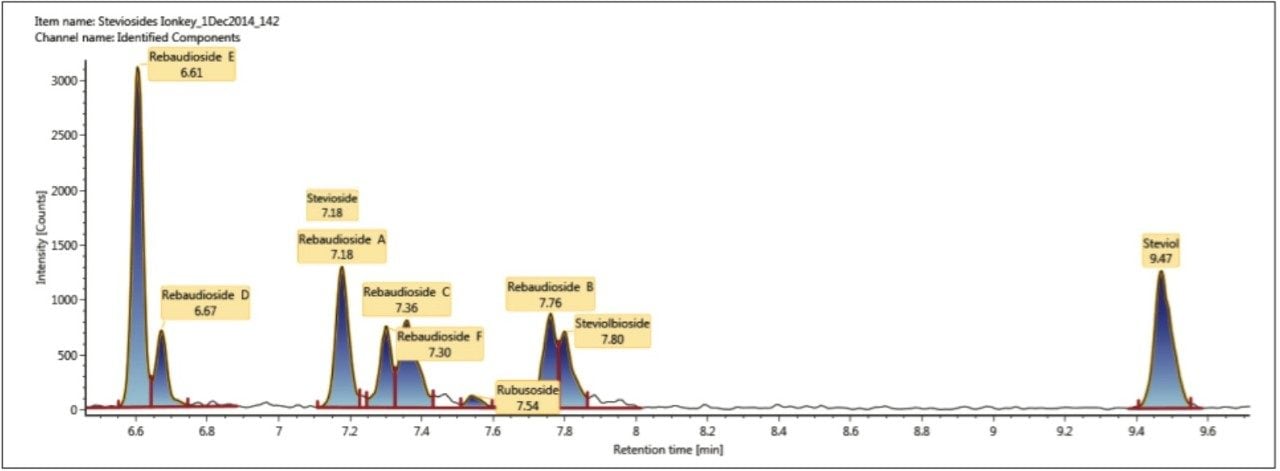
However Figure 6 reveals using ion mobility, that there are two isobaric species at m/z 803.3707 present at retention time 7.18 mins. The retention time aligned multicomponent spectrum at 7.19 mins for rebaudioside A (brown m/z 965.4), and coeluting stevioside (green m/z 803.37) is shown in Figure 7.
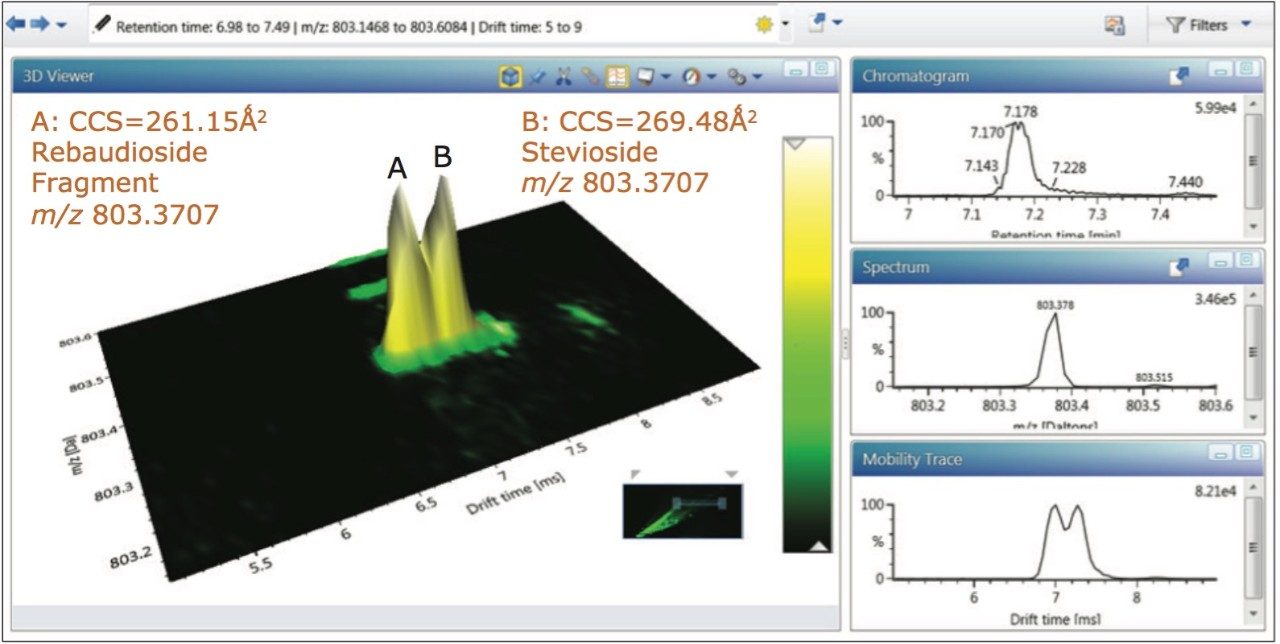
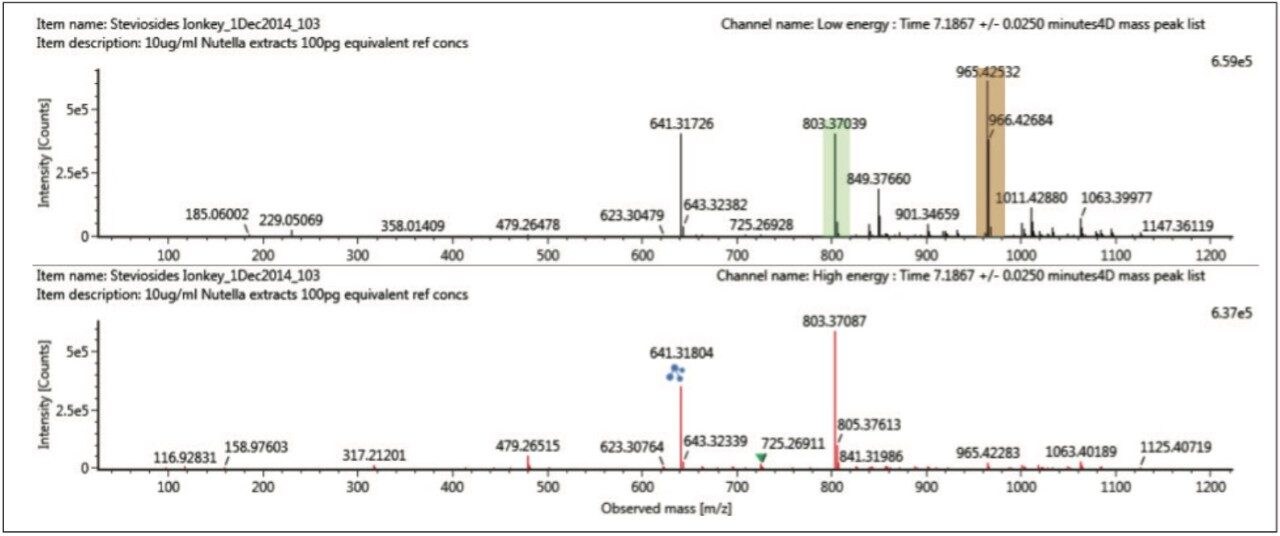
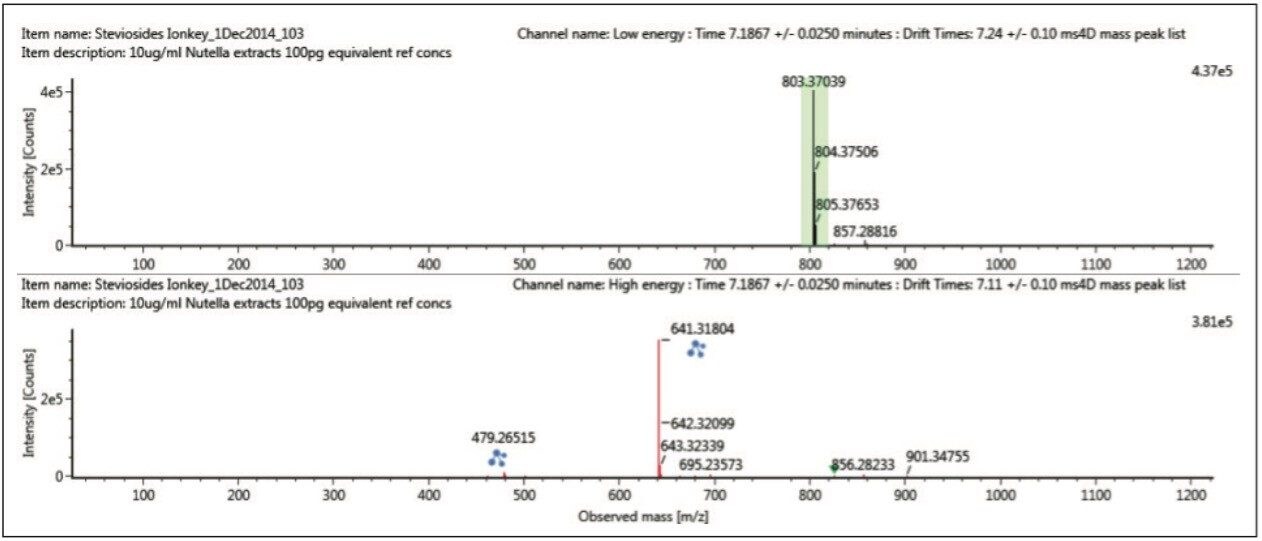
Using the “cleaned up” ion mobility product ion spectra, that are retention and drift time aligned, it is possible to obtain the single component precursor and ion mobility product ion spectra for stevioside (Figure 8) and rebaudioside A (Figure 9) resolved from co-eluting components. Use of ion mobility reveals coelution of isobaric species, which would not have been observed without ion mobility separation. As Figure 6 shows, rebaudioside A has produced an insource fragment ion with a CCS of 261.15 Å,2 compared to 269.48 Å2 for stevioside.
For the first time unique CCS measurements, precursor ion, and corresponding isomer fragmentation spectra for steviol glycosides have been obtained using microfluidic chromatography TWCCSN2 ion mobility screening. This approach reduces the quantity of two expensive commodities, i.e. high purity standards and solvent, and it has the potential to negate the requirement to repeatedly purchase expensive high purity standards, (€2500 for the standards used in this study) for future screening assays.
720005421, February 2016