Among the many stationary phase chemistries available for hydrophilic interaction chromatography (HILIC), zwitterionic materials employing sulfobetaine groups have proven to have the broadest utility. Incorporating negatively and positively charged moieties in a one-to-one molar ratio, zwitterionic stationary phases exhibit strong retention and a net zero surface charge. Here we describe a new zwitterionic sulfobetaine stationary phase based on ethylene-bridged hybrid (BEH) organic/inorganic particles, offering a wider pH range (2–10) than silica-based zwitterionic materials and higher efficiencies compared to organic polymer-based zwitterionic columns. We demonstrate the efficiency, retention, selectivity, batch-to-batch reproducibility, and pH stability of this material. We also show the excellent performance of columns packed with this material for metal-sensitive analytes, which is enabled by the MaxPeak High Performance Surfaces column hardware.
Hydrophilic interaction chromatography (HILIC) is one of the most useful analytical techniques for separating polar analytes.1,2 Employing a polar stationary phase and a less polar mobile phase, retention in HILIC increases with increasing analyte polarity. A range of stationary phases have been used for HILIC, including unbonded silica and materials bearing amide, polyol, amino, sulfobetaine, and other polar functionalities.3,4 Sulfobetaine stationary phases contain negatively-charged sulfonate and positively-charged quaternary ammonium groups in a 1:1 molar ratio, constituting a zwitterion, with a net charge of zero over the pH range of 0–14.5 With their net zero surface charge, zwitterionic stationary phases don’t exhibit the prominent ion-exchange behavior of underivatized silica, which retains cations due to the presence of ionized silanols, and amine-functionalized materials, which retain anions due to ion-exchange with protonated amines.4 Zwitterionic stationary phases have been shown to accumulate a relatively thick layer of adsorbed water, which makes them strongly retentive for polar neutrals in HILIC.6,7 The combination of high retentivity and a net zero surface charge have made zwitterionic columns a popular choice for a wide range of applications, including metabolomics,8 cell culture media analysis,9 pharmaceutical impurity profiling,10 and determinations of the concentrations of toxins in foods.11
Most of the commercially available zwitterionic stationary phases are based on silica particles. Because of the dissolution of silica in basic solutions, these materials are only compatible with mobile phases having a pH in the 2–8 range. A zwitterionic stationary phase based on organic polymer particles is also available and is claimed to be stable from pH 2–10.12 However, the polymer particles are mechanically weaker than silica, limiting their use to pressures below 200 bar (2900 psi).12 Consequently, the size of the polymer particles is limited to 5 μm. Columns packed with polymer particles also exhibit lower efficiencies than those packed with silica-based particles.13 An alternative solution to improve the high pH stability is the use of hybrid organic/inorganic particles. Ethylene-bridged hybrid (BEH) particles have been shown to have good stability in basic mobile phases combined with excellent mechanical strength,14 enabling their use in <2 μm particle sizes for UPLC.15 Here we describe a new sulfobetaine stationary phase based on BEH particles that is stable from pH 2–10, has good batch-to-batch reproducibility, and gives high column efficiencies. The retention and selectivity of this material are described and compared to those of other HILIC stationary phases based on BEH particles. This material is packed into MaxPeak High Performance Surfaces column hardware, which provides improved recovery and peak shape for metal-sensitive analytes by mitigating interactions with metal surfaces.16
The pH values for all buffer solutions were determined as aqueous solutions, and the pH meter was calibrated using aqueous reference buffers. The buffer concentrations indicated below are the concentrations in the aqueous portion of the mobile phase.
The sample contained 120 µg/mL acenaphthene as the hold-up time marker and 80 µg/mL cytosine as the retained analyte, dissolved in the mobile phase. The retention factor of cytosine was 3.3.
|
LC system: |
ACQUITY UPLC I-Class with TUV |
|
Detection: |
254 nm |
|
Column: |
Atlantis Premier BEH Z-HILIC, 1.7 µm, 2.1 x 50 mm (p/n: 186009978) |
|
Column temp.: |
30 °C |
|
Injection volume: |
0.5 µL |
|
Flow rate: |
0.01 to 0.8 mL/min |
|
Mobile phase A: |
80/20 Acetonitrile/100 mM ammonium formate pH 3.0 (aq) |
Six separate samples were used, the first containing 333 µg/mL sodium p-toluenesulfonate (TS) and 333 µg/mL N,N,N-trimethylphenylammonium chloride (TMPA); the second containing 100 µg/mL 3’-deoxyguanosine, 33.3 µg/mL vidarabine, and 33.3 µg/mL 5-methyluridine; the third containing 33.3 µg/mL uridine and 100 µg/mL 2’-deoxyguanosine; the fourth containing 33.3 µg/mL adenosine and 33.3 µg/mL 2’-deoxyuridine; the fifth containing 1.0 mg/mL toluene (the hold-up time marker); and the sixth containing 33.3 µg/mL uridine and 1.0 mg/mL toluene. All samples were dissolved in the mobile phase. For all analytes, except TS and TMPA, a mobile phase containing 90/10 acetonitrile/20 mM ammonium acetate pH 4.7 (aq) was used. TS and TMPA were separated using a 90/10 acetonitrile/100 mM ammonium acetate pH 4.7 (aq) mobile phase.
|
LC system: |
ACQUITY UPLC H-Class with PDA |
|
Detection: |
254 nm |
|
Columns: |
Atlantis Premier BEH Z-HILIC, 1.7 µm, 2.1 x 50 mm (p/n: 186009978); ACQUITY UPLC BEH Amide, 1.7 µm, 2.1. x 50 mm (p/n: 186004800); ACQUITY UPLC BEH HILIC, 1.7 µm, 2.1 x 50 mm (p/n: 186003460) |
|
Column temp.: |
30 °C |
|
Injection volume: |
3.0 µL |
|
Flow rate: |
0.2 mL/min |
|
Mobile phase A: |
90/10 Acetonitrile/20 mM ammonium acetate pH 4.7 (aq) or 90/10 Acetonitrile/100 mM ammonium acetate pH 4.7 (aq) |
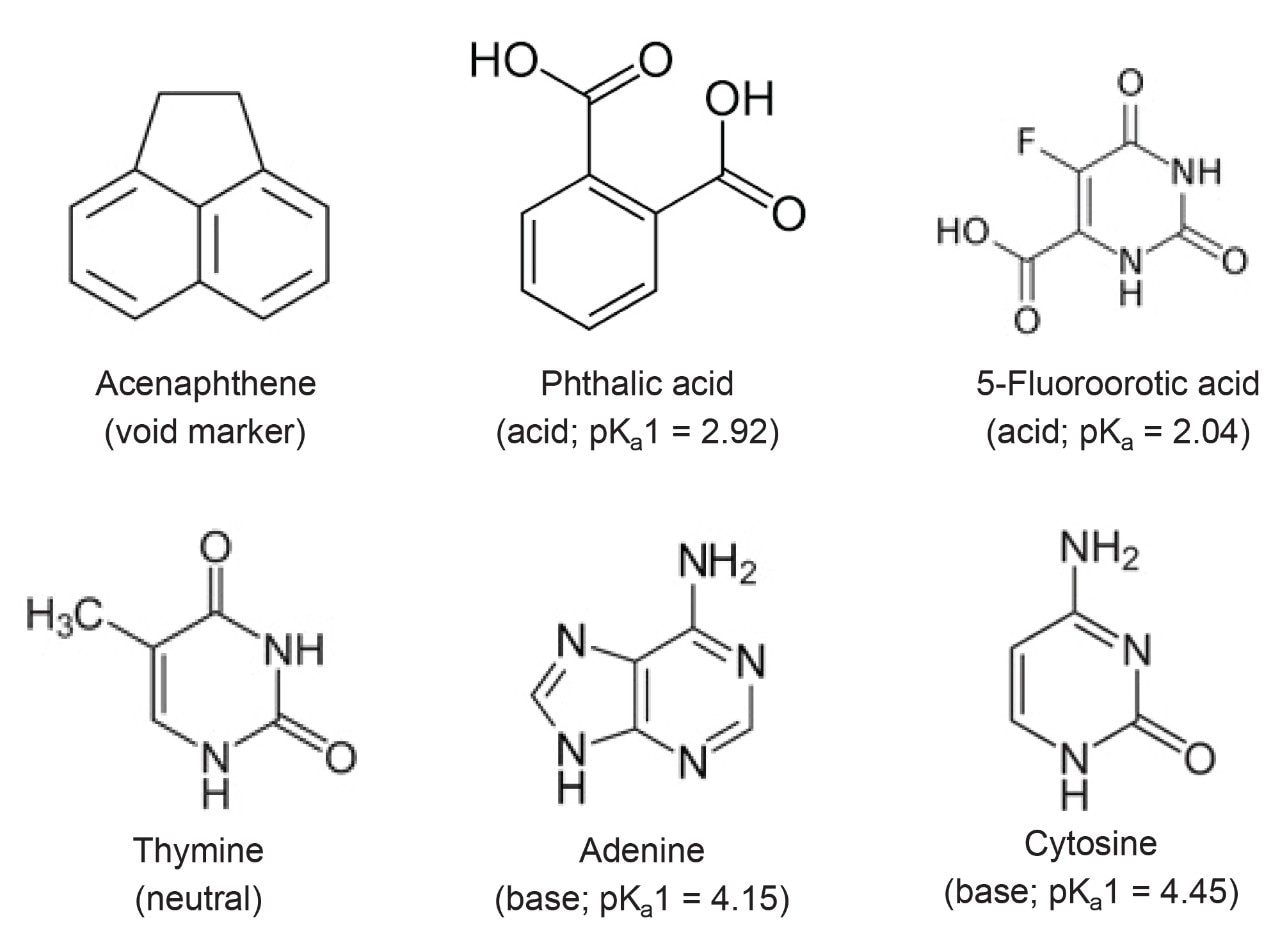
The sample contained 19 µg/mL acenaphthene, 3.7 µg/mL thymine, 25 µg/mL phthalic acid, 3.7 µg/mL adenine, 7.7 µg/mL cytosine, and 25 µg/mL 5-fluoroorotic acid, dissolved in the mobile phase.
|
LC system: |
ACQUITY UPLC with TUV |
|
Detection: |
254 nm |
|
Columns: |
Atlantis Premier BEH Z-HILIC, 1.7 µm, 2.1 x 50 mm (p/n: 186009978) |
|
Column temp.: |
30 °C |
|
Injection volume: |
3.0 µL |
|
Flow rate: |
0.5 mL/min |
|
Mobile phase A: |
90/10 Acetonitrile/100 mM ammonium formate pH 3.0 (aq) |
The sample contained 19 µg/mL acenaphthene, 3.7 µg/mL adenine, and 7.7 µg/mL cytosine, dissolved in 95/5 acetonitrile/100 mM ammonium formate pH 3.0 (aq). After separating this sample using a 95/5 acetonitrile/100 mM ammonium formate pH 3.0 (aq) mobile phase, 75 column volumes of 60/40 acetonitrile/10 mM ammonium bicarbonate pH 11.0 (aq) were passed through the column followed by washing with 50/50 acetonitrile/water, 10/90 acetonitrile/water, then equilibrating with the 95/5 acetonitrile/100 mM ammonium formate pH 3.0 (aq) test mobile phase. This cycle was repeated 100 times, with the column temperature maintained at 70 °C throughout the test.
|
LC system: |
ACQUITY UPLC H-Class with PDA |
|
Detection: |
254 nm |
|
Column: |
Atlantis Premier BEH Z-HILIC, 1.7 µm, 2.1 x 50 mm (p/n: 186009978) |
|
Column temp.: |
70 °C |
|
Injection volume: |
1.0 µL |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
Water |
|
Mobile phase B: |
Acetonitrile |
|
Mobile phase C: |
100 mM Ammonium formate pH 3.0 (aq) |
|
Mobile phase D6: |
60/40 Acetonitrile/10 mM ammonium bicarbonate pH 11.0 (aq) |
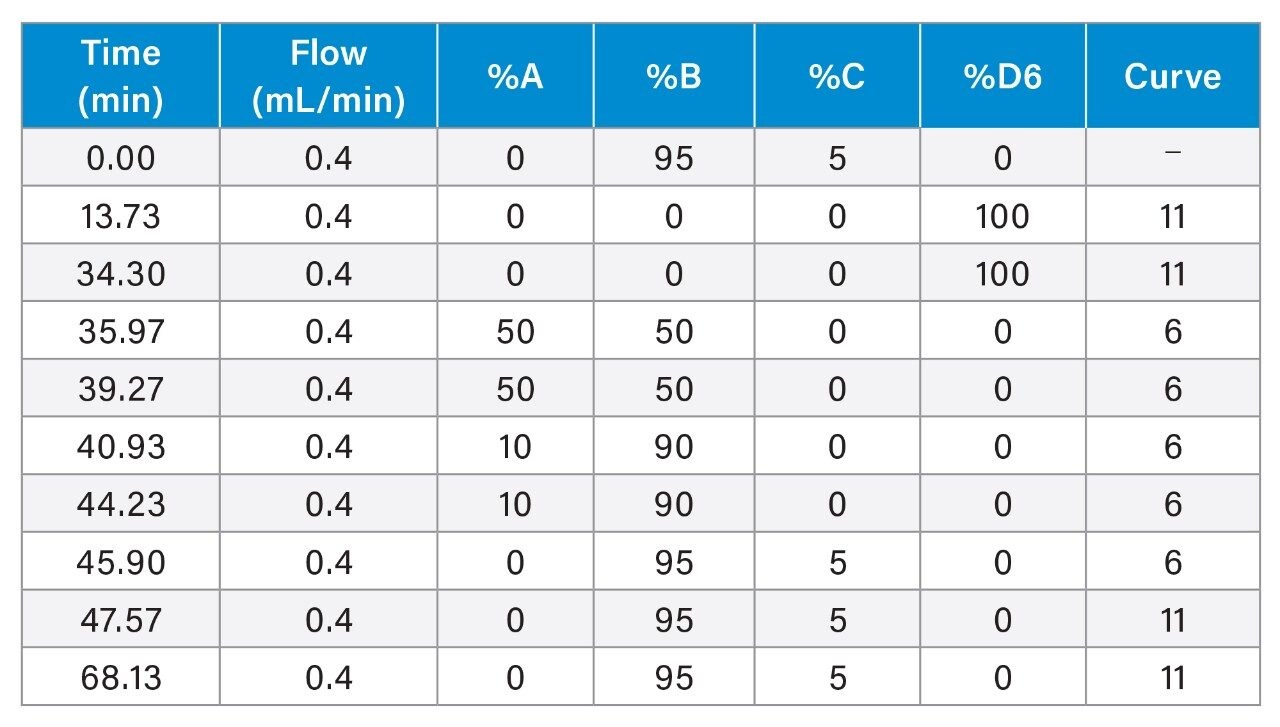
The sample contained 19 µg/mL acenaphthene, 3.7 µg/mL adenine, and 7.7 µg/mL cytosine, dissolved in the mobile phase.
|
LC system: |
ACQUITY UPLC H-Class with PDA |
|
Detection: |
254 nm |
|
Column: |
Atlantis Premier BEH Z-HILIC, 1.7 µm, 2.1 x 50 mm (p/n: 186009978) |
|
Column temp.: |
70 °C |
|
Injection volume: |
1.0 µL |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase B: |
Acetonitrile |
|
Mobile phase C: |
100 mM Ammonium formate pH 2.0 (aq) |
|
Isocratic composition: |
90/10 B/C |
Separate samples containing 50 µg/mL each of adenosine monophosphate disodium salt, adenosine diphosphate disodium salt hydrate, and adenosine triphosphate disodium salt hydrate dissolved in the mobile phase were used.
|
LC system: |
ACQUITY Premier BSM with PDA |
|
Detection: |
260 nm |
|
Columns: |
Atlantis Premier BEH Z-HILIC, 1.7 µm, 2.1 x 50 mm (p/n 186009978), a standard column packed with Atlantis BEH Z-HILIC, 1.7 µm |
|
Column temp.: |
30 °C |
|
Injection volume: |
0.4 µL |
|
Flow rate: |
0.5 mL/min |
|
Mobile phase A: |
70/30 Acetonitrile/20 mM ammonium acetate pH 6.8 (aq) |
|
Chromatography software: |
Empower 3 FR4 |
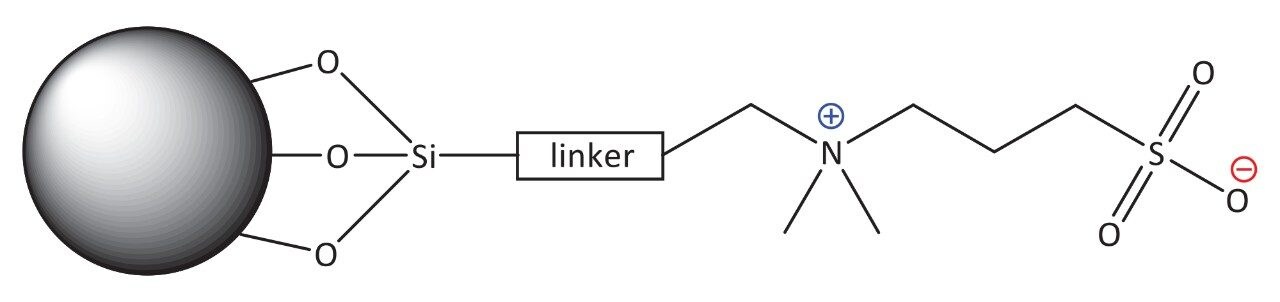
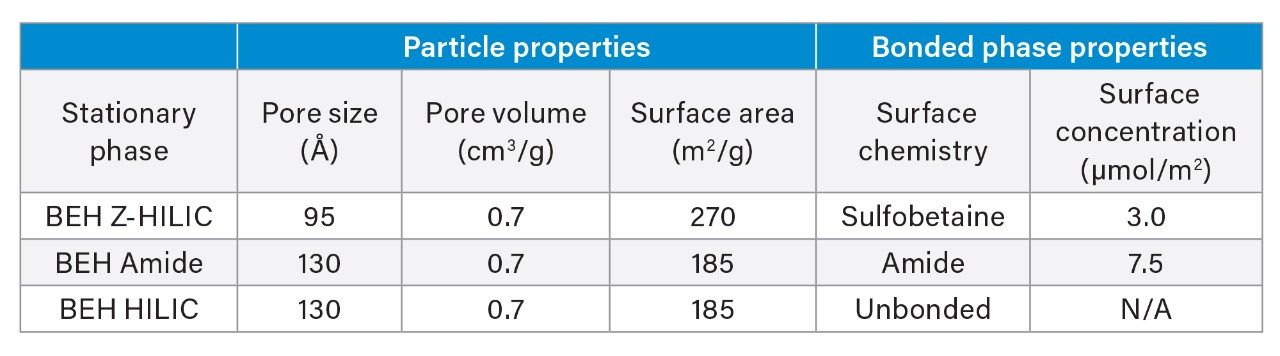
The Atlantis BEH Z-HILIC stationary phase is based on BEH particles with a pore size of 95 Å, which has an approximately 50% higher surface area than the 130 Å particles used in the other HILIC stationary phases based on BEH particles. The higher surface area of the 95 Å particles results in greater retention. The particles are derivatized with sulfobetaine groups, giving the surface structure shown in Figure 1. Table 1 summarizes the key properties of the BEH Z-HILIC stationary phase compared to those of BEH Amide and BEH HILIC.
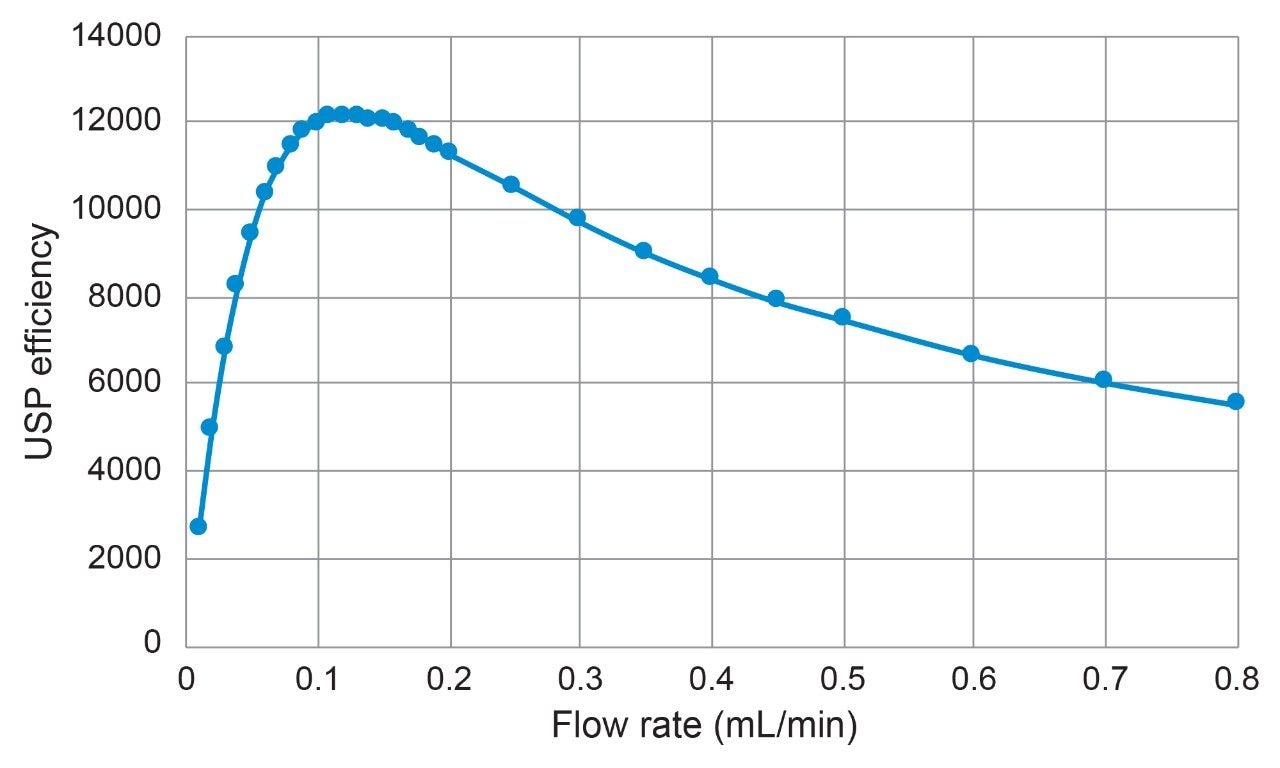
Columns packed with 1.7 μm BEH Z-HILIC exhibit high efficiencies, comparable to those of 1.7 μm BEH HILIC and BEH Amide Columns. Shown in Figure 2 is a plot of efficiency versus flow rate for an Atlantis Premier BEH Z-HILIC, 1.7 µm, 2.1 x 50 mm Column. The results indicate that a maximum USP efficiency of 12,100 was achieved for cytosine at a flow rate of 0.13 mL/min. This is equivalent to a plate height of 4.1 µm, a reduced plate height of 2.4, and an efficiency per unit length of 242,000 plates/m. Similar plate heights have previously been reported for BEH HILIC, 1.7 µm Columns.17,18
To evaluate the retention and selectivity of the Z-HILIC stationary phase, we used tests described by Kawachi
et al.19 These tests employ a set of analytes chosen to probe different types of selectivity. The structures of the analytes are shown in Figure 3. The relative retention (r) of uridine vs 5-methyl uridine is a measure of the methylene group selectivity, that of uridine vs 2’-deoxyuridine is an indicator of the hydroxyl group selectivity, r(vidarabine/adenosine) probes the configurational isomer selectivity, r(2’-deoxyguanosine/ 3’-deoxyguanosine) shows the regioisomer selectivity, r(trimethylphenylammonium chloride/uridine) indicates the cation-exchange selectivity, and r(sodium p-toluenesulfonate/uridine) probes the anion-exchange selectivity.
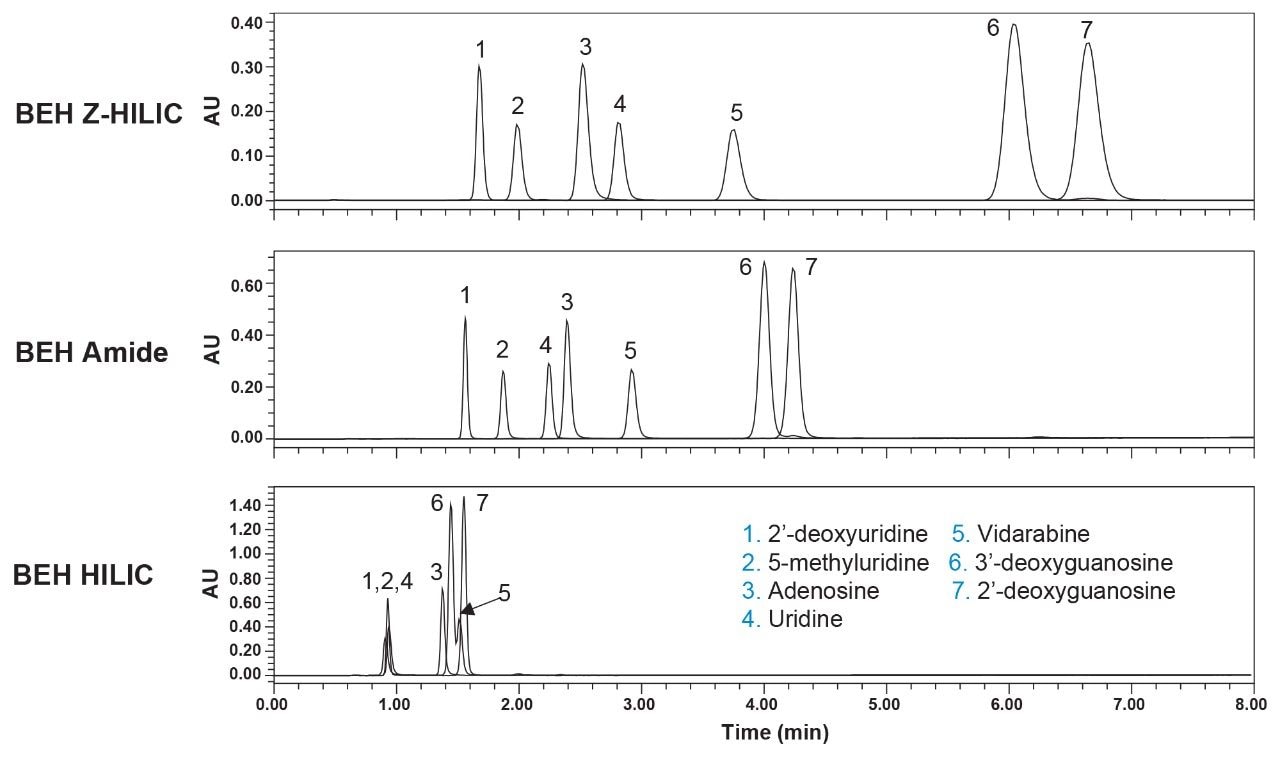
Shown in Figure 4 is a comparison of the chromatograms obtained for seven of these analytes using BEH Z-HILIC, BEH Amide, and BEH HILIC Columns. The results demonstrate that the Z-HILIC Column gives the highest retention times for all seven compounds, while the BEH HILIC Column gives the lowest. The retention factors for uridine and the selectivity factors for the key analyte pairs are summarized in Table 2. The results show that the Z-HILIC material has the highest retention factor for uridine and the relative retentions for methylene groups, hydroxyl groups, and the configurational isomers vidarabine and adenosine.
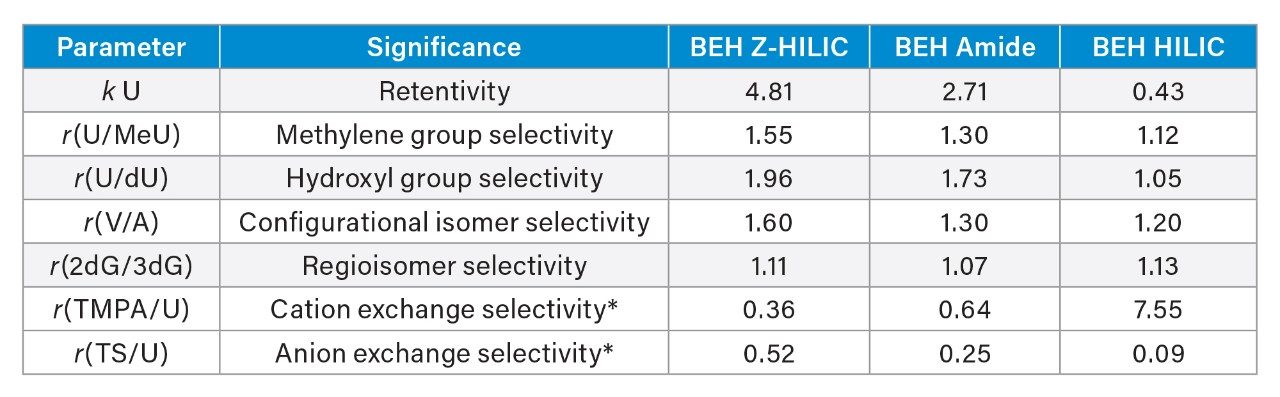
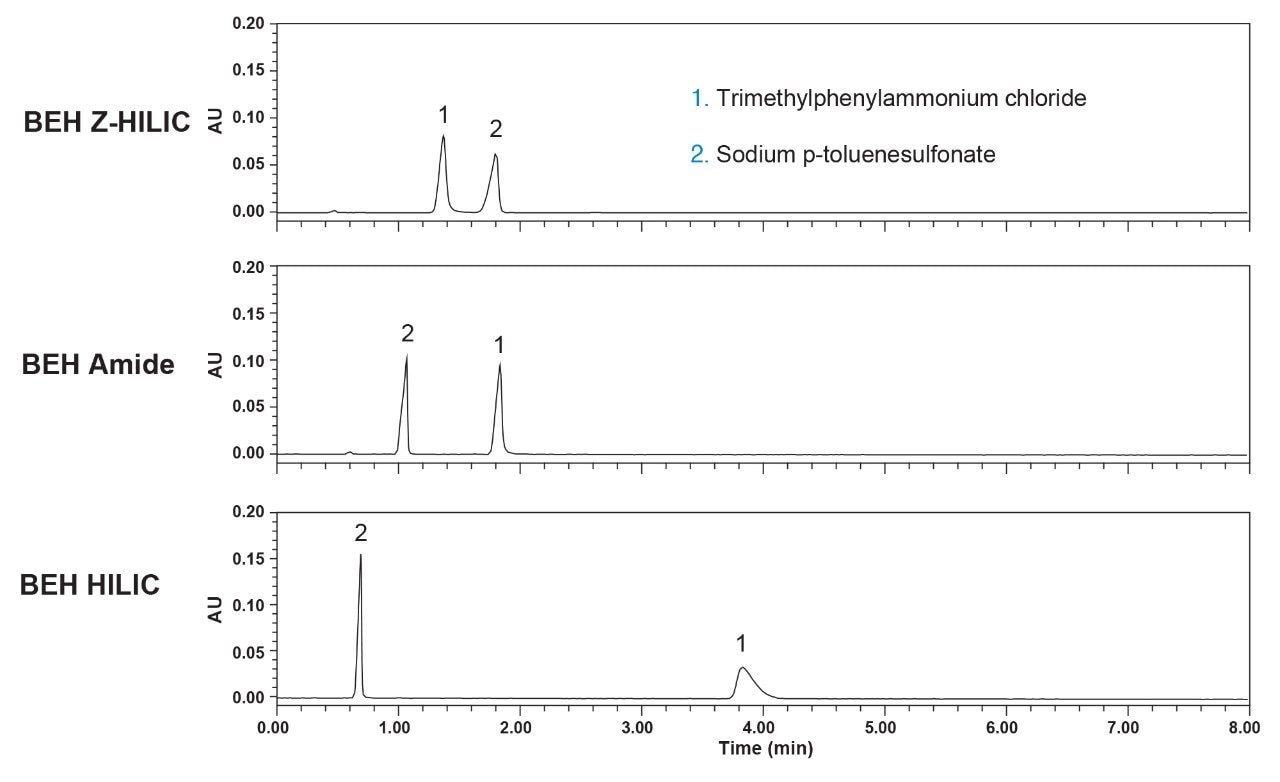
The selectivities for the two ionized compounds, trimethylphenylammonium chloride (TMPA) and sodium p-toluenesulfonate (TS), reveal the largest differences between the columns, as shown in Figure 5. The Z-HILIC Column gives greater retention for the anion TS than do the BEH Amide and unbonded BEH Columns, while the opposite is true for the cation TMPA. This indicates that the Z-HILIC stationary phase has a more positive surface charge than the BEH Amide and unbonded BEH materials under these separation conditions. The BEH HILIC stationary phase shows the greatest retention of TMPA, consistent with a negative surface charge due to ionization of surface silanols on the unbonded BEH particles. The differences in selectivity between these three stationary phases makes them a useful set to screen during method development.
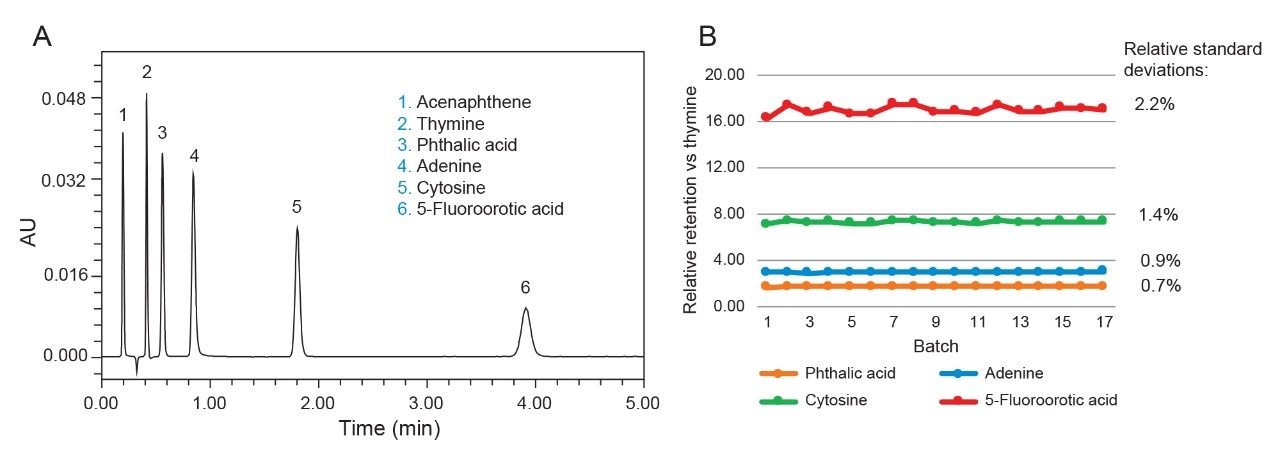
The synthesis of Atlantis BEH Z-HILIC involves the surface modification of 95 Å BEH particles using a polymer grafting approach. By carefully controlling the surface modification process, excellent chromatographic reproducibility has been achieved. To evaluate the batch-to-batch reproducibility, we used an isocratic separation of a mixture of five analytes, comprising acidic, basic, and neutral compounds (see Figure 6). A representative separation of this mixture using a 1.7 μm Atlantis Premier BEH Z-HILIC Column is shown in Figure 7A. The results obtained for seventeen different batches of Atlantis BEH Z-HILIC, 1.7 µm exhibit very similar separations, as demonstrated by the relative retention chart displayed in Figure 7B. The relative standard deviation (RSD) for the retention factor of thymine was 2.0% and the RSDs for the relative retentions of the other analytes vs thymine ranged from 0.7 to 2.2%. These RSD values are like those of the most reproducible C18 columns.20
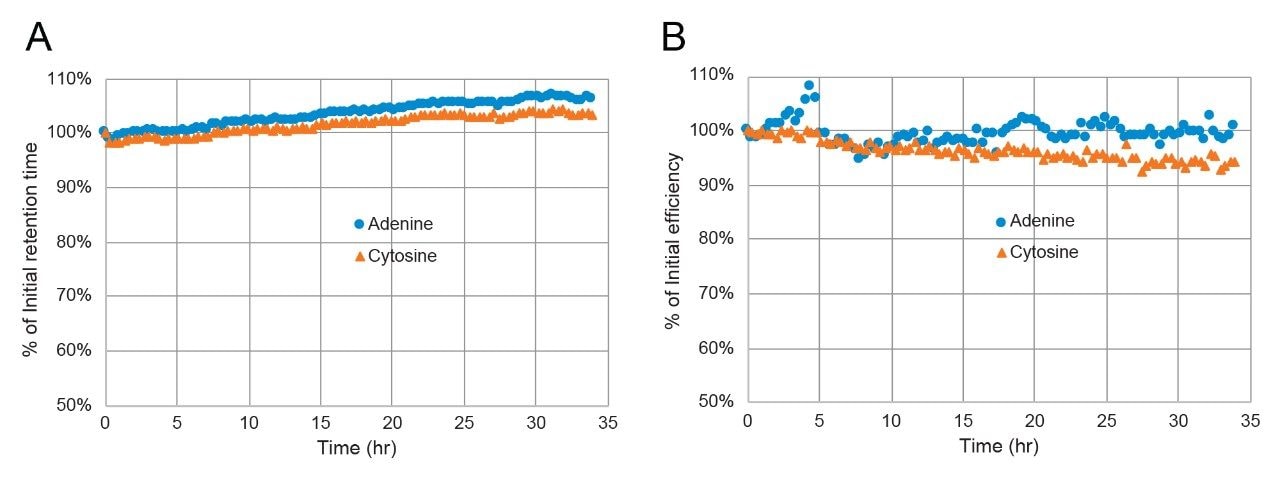
To evaluate the acid and base stability of Atlantis Premier BEH Z-HILIC Columns, accelerated testing at elevated temperatures was used. The base stability evaluation employed a challenge mobile phase containing 60/40 acetonitrile/10 mM ammonium bicarbonate pH 11.0 (aq) and a temperature of 70 °C. The column efficiency and retention were characterized by separating a mixture containing acenaphthene, adenine, and cytosine using a 95/5 (v/v) acetonitrile/100 mM ammonium formate pH 3.0 (aq) mobile phase. After exposure to 75 column volumes of the challenge mobile phase, the column was washed with 50/50 acetonitrile/water, then 90/10 acetonitrile/water before equilibrating with the efficiency/retention test mobile phase. This test-challenge-wash-equilibrate cycle was repeated 100 times. Silica-based HILIC columns show large efficiency losses when subjected to this test, due to dissolution of the silica particles caused by the pH 11 mobile phase.21 However, columns packed with BEH-based stationary phases show excellent stability under these conditions. The dependence of efficiency and retention on the time exposed to the pH 11 mobile phase for an Atlantis Premier BEH Z-HILIC, 1.7 µm Column is shown in Figure 8. After 34 hours of exposure the column showed a 5.8% decrease in efficiency for cytosine and 2.5-5.0% increases in retention time. Based on these results, the recommended upper limit for Atlantis Premier BEH Z-HILIC Columns is pH 10.
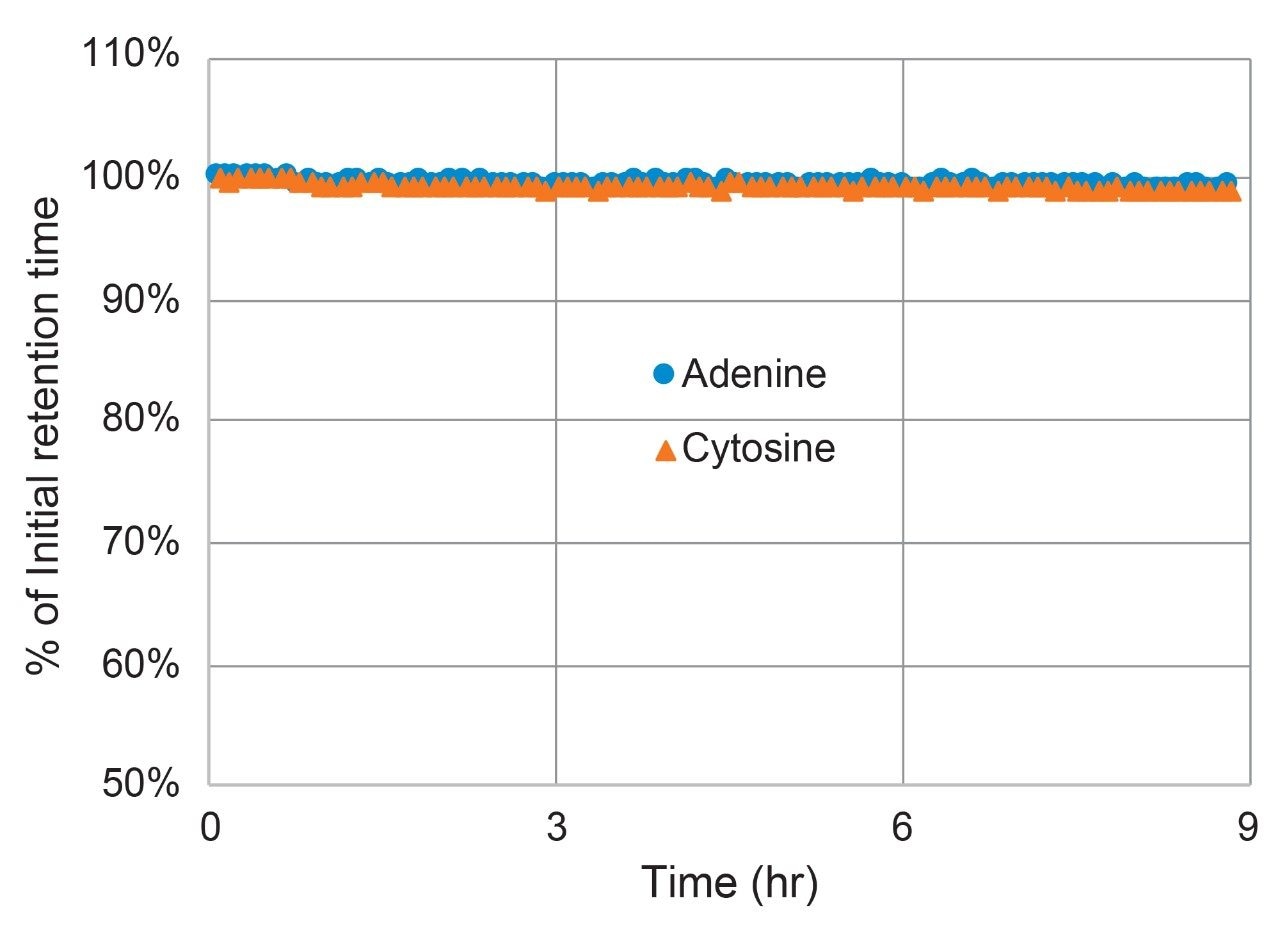
The acid stability of the Atlantis BEH Z-HILIC stationary phase was assessed using a 90/10 acetonitrile/100 mM ammonium formate pH 2.0 (aq) mobile phase and a temperature of 70 °C. The condition of the column was monitored by separating a mixture of acenaphthene, adenine, and cytosine using the same mobile phase. Exposure to acidic mobile phases can result in hydrolysis of bonded phases, which leads to changes in retention and selectivity.21 The results obtained for an Atlantis Premier BEH Z-HILIC Column are shown in Figure 9 as a plot of the percent of the initial retention times for adenine and cytosine vs the time exposed to the mobile phase. After 9 hours of exposure, the retention times decreased less than 1%. Based on these results, the recommended lower limit for Atlantis Premier BEH Z-HILIC Columns is pH 2.
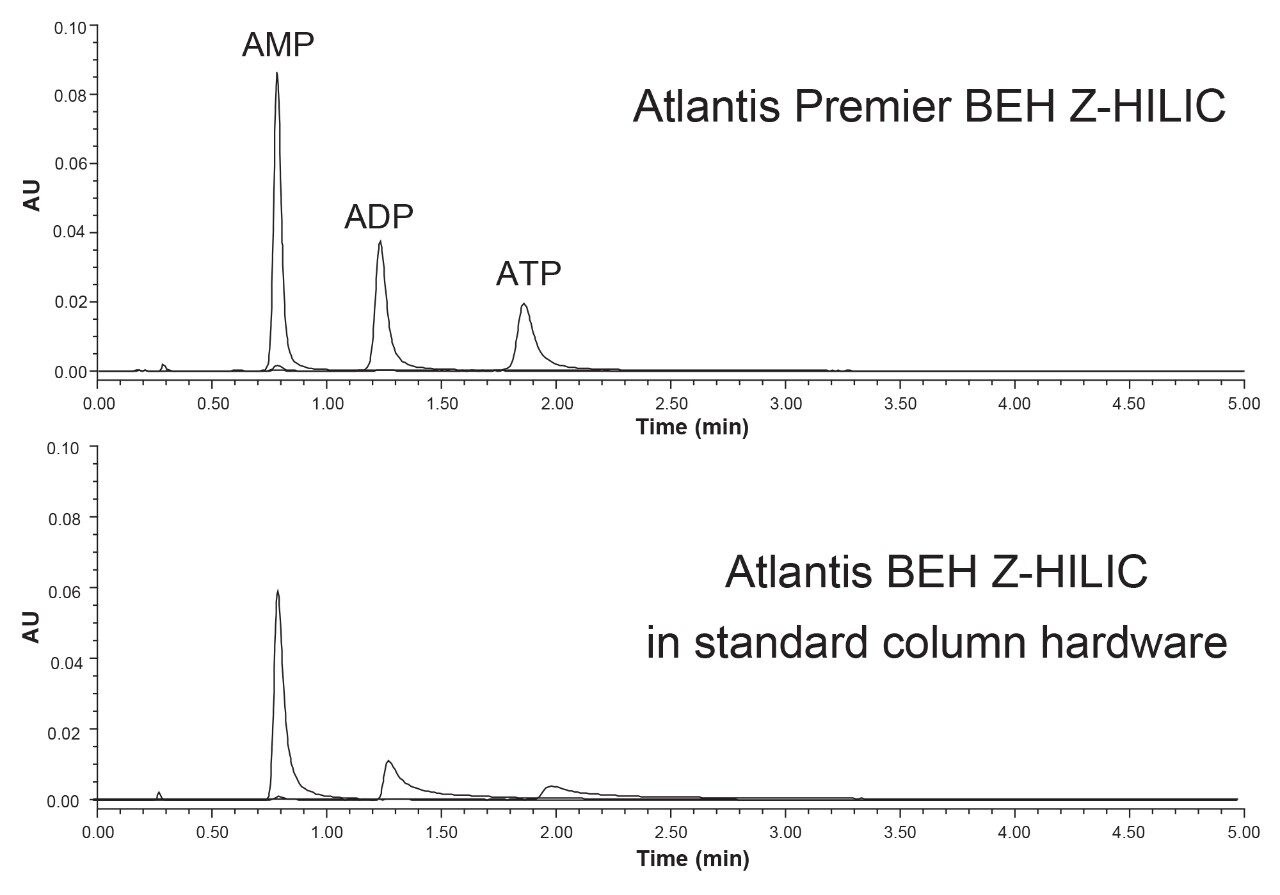
Many polar compounds, particularly those that contain two or more carboxylate and/or phosphate groups, can interact with the stainless-steel surfaces in HPLC columns.22 The effects of these interactions range from peak broadening and tailing to complete loss of analyte signal. To mitigate these interactions, we used column hardware for Atlantis Premier BEH Z-HILIC that has been modified using MaxPeak High Performance Surfaces (HPS).16 The dramatic improvement afforded by this column hardware is shown in Figure 10. Adenosine monophosphate (AMP), adenosine diphosphate (ADP), and adenosine triphosphate (ATP) were analyzed using an Atlantis Premier BEH Z-HILIC Column and a column packed with the same stationary phase, but employing standard hardware. With the standard hardware, ADP and ATP were detected as broad, tailing peaks. However, when using the Atlantis Premier BEH Z-HILIC Column (employing MaxPeak HPS hardware) all three nucleotides were observed as symmetric peaks having the expected areas. This evaluation was carried out using an ACQUITY Premier System, in which MaxPeak HPS Technology has been used to mitigate adsorption in the UPLC instrument.23
These results highlight the key characteristics of Atlantis Premier BEH Z-HILIC, 1.7 µm Columns. Packed with ethylene-bridged hybrid particles that have been derivatized with sulfobetaine groups, these columns exhibit high efficiencies, strong retention for polar analytes, complementary selectivity to existing BEH-based HILIC chemistries, excellent batch-to-batch reproducibility, stability from pH 2–10, and outstanding performance for metal-sensitive compounds. This combination of attributes makes these columns useful for a wide range of applications, including metabolomics, cell culture media analysis, pharmaceutical impurity analysis, and food testing.
720007311, Revised December 2021