
This application note describes the transfer of existing HPLC methods to the Waters ACQUITY UPLC System. Standard solutions were used to assess chromatography as there will not be changes to the extraction of metabolites from edible tissues1 or honey.2
The four drugs furazolidone, furaltadone, nitrofurazone, and nitrofurantoin are veterinary drugs that belong to the family of nitrofuran antibiotics and are banned in meat destined for human consumption.3
The four drugs are readily metabolized in livestock to undetectable levels, metabolizing to AOZ, AMOZ, SCA, and AHD, respectively. Their metabolites are more persistent in edible tissues and these can be used to detect use of the parent drug. Proportions of these metabolites exist as protein adducts and the established method of analysis contains acid hydrolysis and derivatisation steps prior to extraction.4
This method transfer will determine the suitability of UPLC for the determination of these residues. Previous methods1 have employed HPLC-MS/MS using a Waters Alliance 2795 System with a Waters Quattro Premier XE Mass Spectrometer. The ACQUITY UPLC has been designed to significantly reduce run times using novel column chemistries. ACQUITY UPLC also gives advantages over regular HPLC in sensitivity, specificity, accuracy, and resolution.

A solution containing the four derivatized metabolites and their deuterated internal standards, 2-NPAMOZ-D5, 2-NP-SCA-13C1 15N2, and 2-NP-AHD13C3 was produced at a concentration of 8 ng/mL (equivalent to 2 ppb in animal tissue) in 1:4 methanol: water (v/v). This solution was further diluted to 4, 2, 0.4, and 0.2 ng/mL (equivalent to 1, 0.5, 0.1, and 0.05 ppb in extracted samples1).
A standard curve was created for all compounds and the chromatography was assessed for each compound at each level. All concentrations reported in this technical note refer to the derivitized metabolite.
Mobile phase A was 1:4 methanol: water (v/v) + 0.5 mM ammonium acetate. Mobile phase B was 9:1 methanol: water (v/v) + 0.5 mM ammonium acetate. The column used was an ACQUITY UPLC BEH C18 1.7 μm 2.1 x 100 mm. A 50 μL injection was used with a flow rate of 0.45 mL/min.
The gradient used is described below:
0.00 min 5% mobile phase B (hold 0.2 min)
2.00 min 50% mobile phase B
2.02 min 100% mobile phase B (hold 1.1 min)
Total run time was 4.00 minutes.
The Quattro Premier XE was set up in positive ion mode using an electrospray source. All compounds were optimized and two Multiple Reaction Monitoring (MRM) transitions were obtained for each derivatized metabolite and one for each internal standard. Two MRM transitions were monitored for each derivatized metabolite in accordance with European Union guidelines.5 The primary transition is used for quantification and the secondary transition is used for conformation purposes. These MRM transitions are listed in Table 1 along with their respective cone voltages and collision energies.
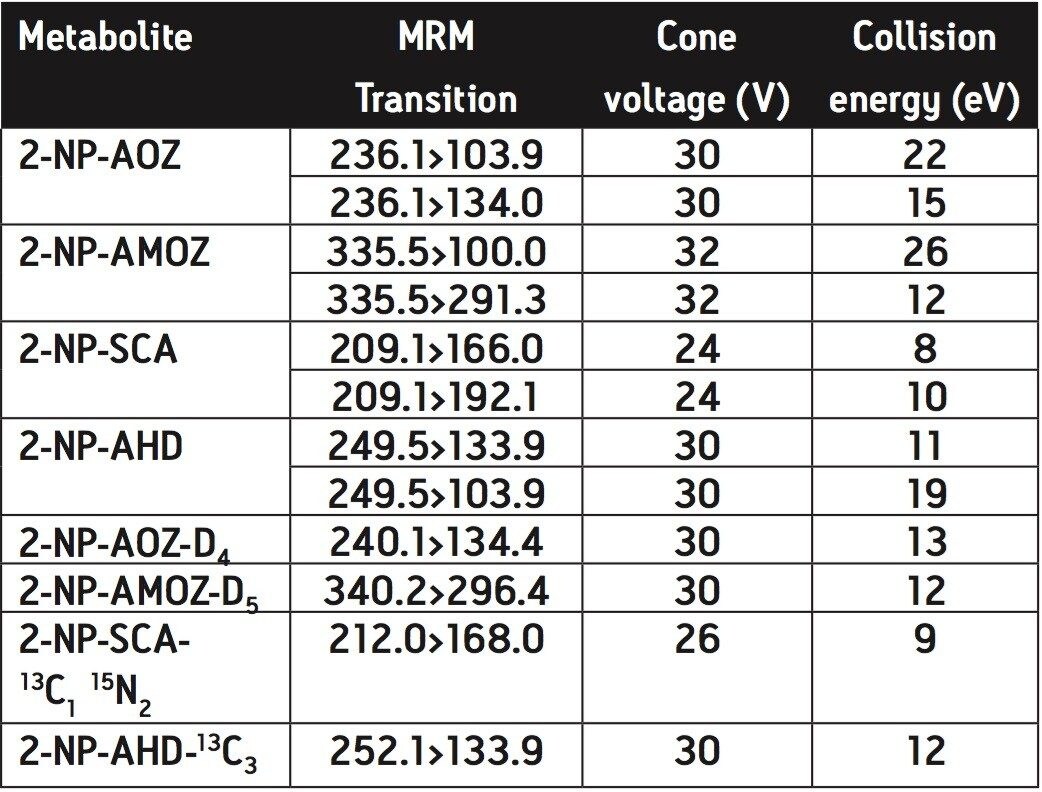
A dwell time of 0.025 s was chosen for this analysis. This gave approximately 14 points per chromatographic peak.
The following electrospray parameters were used on the Quattro Premier XE:
|
Capillary voltage: |
3 kV |
|
Extractor: |
4 V |
|
RF Lens: |
0.5 V |
|
Source temperature: |
120 °C |
|
Desolvation gas temperature: |
400 °C |
|
Desolvation gas (nitrogen): |
900 L/hr |
|
Cone gas (nitrogen): |
20 L/hr |
|
Collision cell gas (argon): |
3.3 10-3 mBar |
Data were acquired with Waters MassLynx Software and processed with Waters TargetLynx Application Manager.
Sensitivity was achieved for all components of the derivatized metabolite mixture at 0.4 ng/mL (equivalent to 0.1 ppb). This is ten times below the EU minimum required performance limit (MPRL) guideline.
Figures 1, 2, and 3 show typical chromatography obtained using ACQUITY UPLC. The novel technology offered by UPLC gives the advantage of a run time of four minutes, compared to the traditional HPLC run time of approximately 10 minutes.
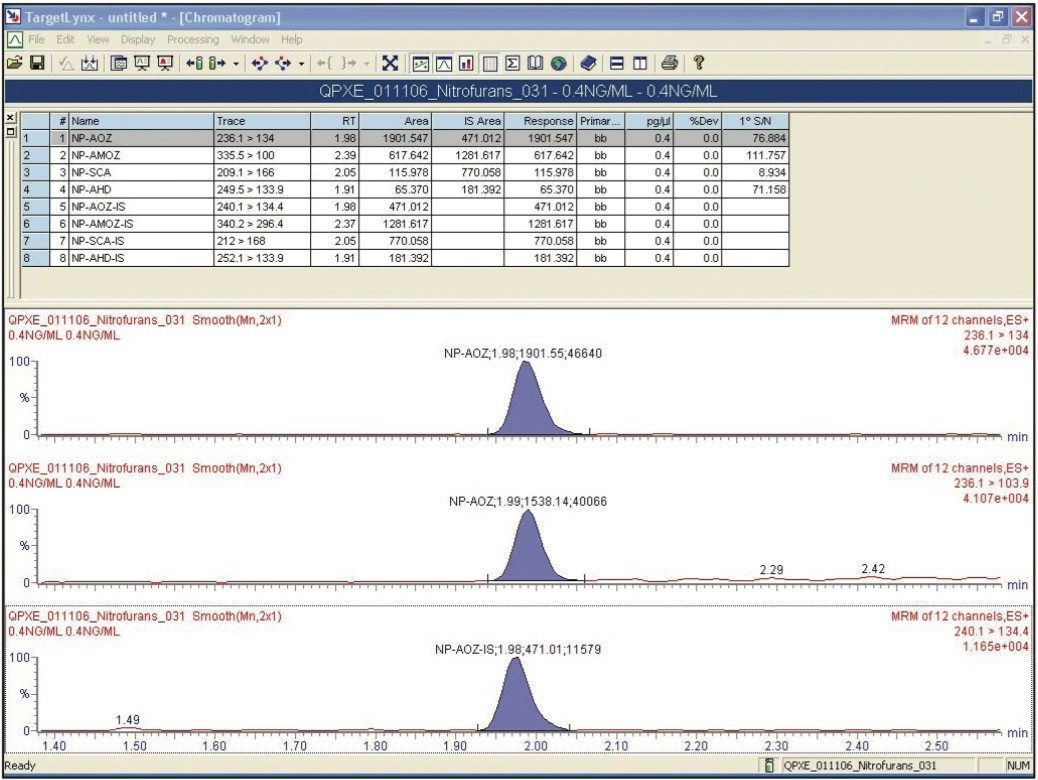

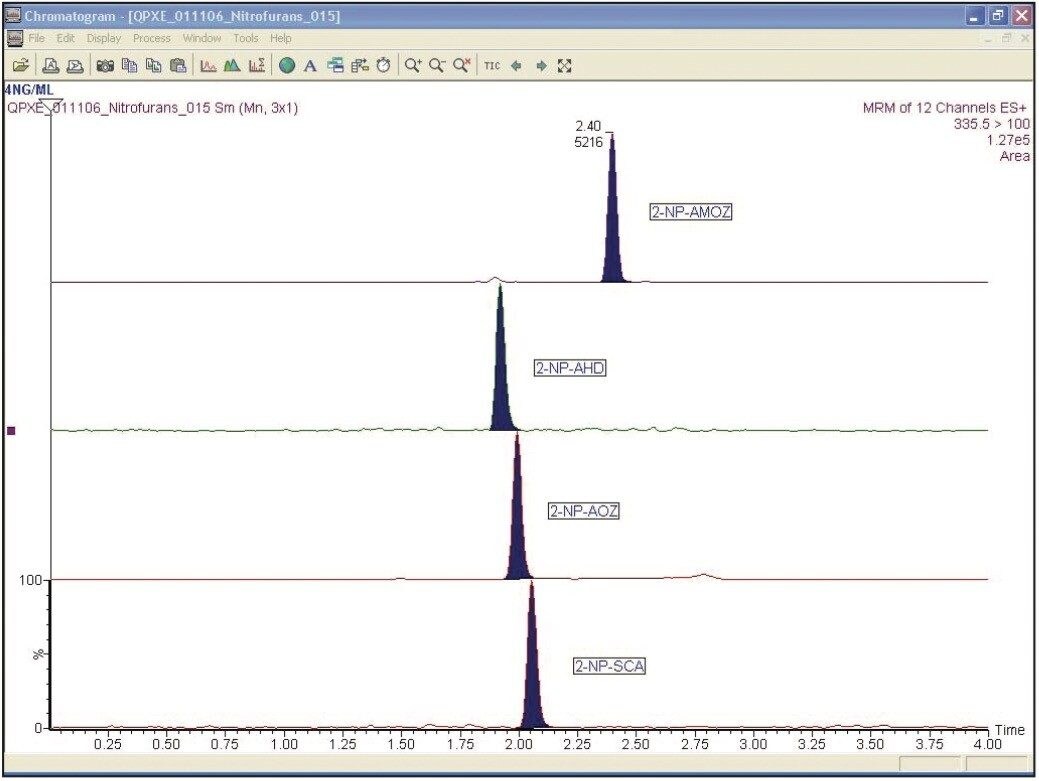
When assessing the Signal to Noise (S:N) ratio’s for all derivatized nitrofuran metabolites it is clear that they have differing response factors. An acceptable limit of detection was allowed to be a S:N ratio of 3:1. All derivatized nitrofuran metabolites have a S:N ratio significantly greater than this value at 0.4 ng/mL (equivalent to 0.1 ppb). Figures 2 and 3 show the chromatographic separation of the four derivatized nitrofuran metabolites with a four minute run time.
The established HPLC method for the determination of nitrofuran veterinary drug residues has been successfully transferred to a UPLC coupled to a Quattro Premier XE Mass Spectrometer. The new UPLC method provides a two fold increase in the speed of separation over the HPLC method with excellent sensitivity that allows detection at ten times below the EU minimum required performance limit (MPRL) guideline.
720001951, September 2007