For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
This application brief quickly and accurately determines the spatial resolution of target compounds within biological tissues.
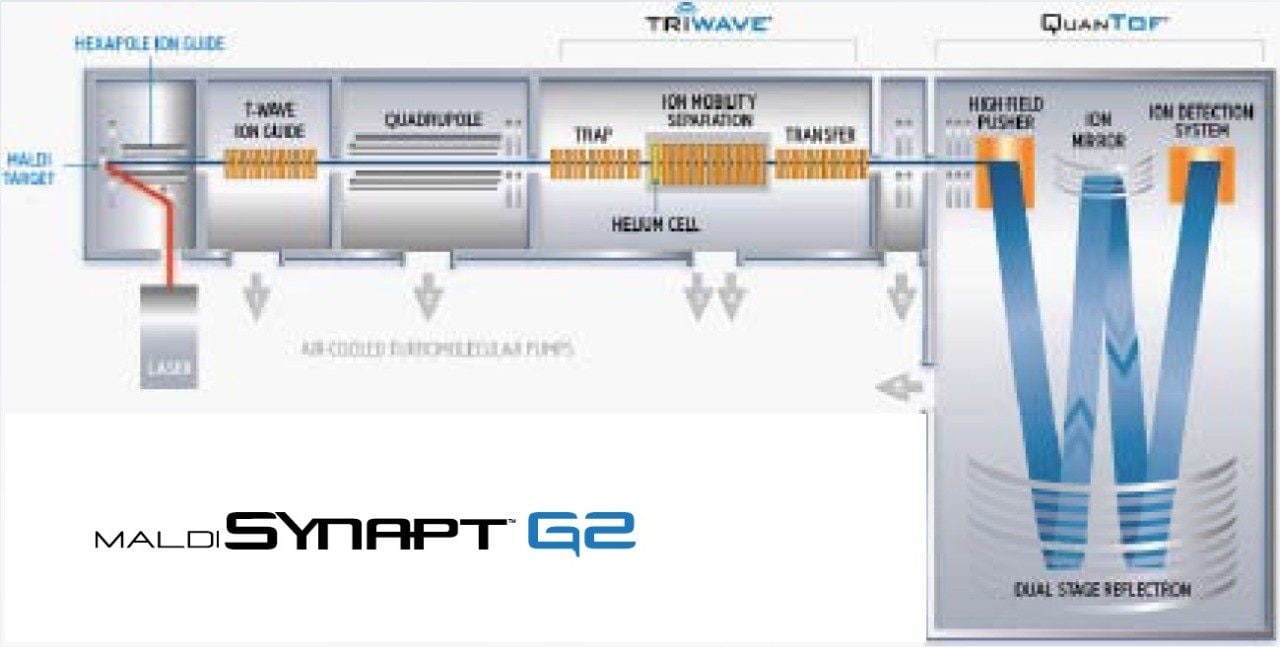
The MALDI SYNAPT G2 HDMS System allows MALDI imaging experiments to be performed rapidly with the use of increased laser repetition rates, and the spatial distribution of analytes to be determined more confidently using a small laser spot profile.
There has been a rapid increase in interest of mass spectral imaging directly from tissue over the last several years; the use of MALDI imaging has continued to expand1. The main driver behind development of instrumentation has been in reducing data acquisition time through the use of higher repetition rate lasers, while reducing the laser spot diameter (size of the pixel) to achieve higher spatial resolution.
The MALDI SYNAPT G2 HDMS System provides an ideal platform to conduct MALDI imaging studies through its high sensitivity, selectivity, and speed. Uniquely, it provides the ability to acquire high resolution, exact mass data across a wide mass range at high acquisition rates, while providing the ability to separate target analytes from isobaric background interferences using gas-phase ion mobility separations2. Here we demonstrate the practical impact of using a solid state laser with pulse repetition rates of 1 kHz and variable spot size (200 μm or 60 μm), now available on the MALDI SYNAPT G2 HDMS System.
Thin (~15 μm) sections of rat lung and brain tissue were produced using a cryotome and thaw-mounted onto standard microscope slides. Several coats of α-cyano-4-hydroxycinnamic acid (CHCA) matrix were then deposited onto the prepared samples using an airbrush and allowed to dry before the sample plates were loaded into the instrument.
Data was acquired from a MALDI SYNAPT G2 HDMS System equipped with a diode pumped frequency tripled Nd:YAG UV laser (355 nm wavelength, 50 μJ pulses of <3 ns duration) operating at 1 kHz. Ion images were produced using BioMap (Novartis) software.
The laser was focused to a spot size of approximately 60 μm. Mass spectra were acquired in a raster mode where the sample plate was moved in 20 μm increments representing individual pixels. MS data were accumulated for a period of 300 ms (300 laser shots) into each pixel (spectrum). MS spectra from over 200,000 pixels were obtained in order to create MALDI ion images of the sample over an area of approximately 8 mm x 11 mm (400 pixels x 550 pixels). The localization of different ions within the tissue section could be visualized with high spatial resolution, as shown in Figure 2.
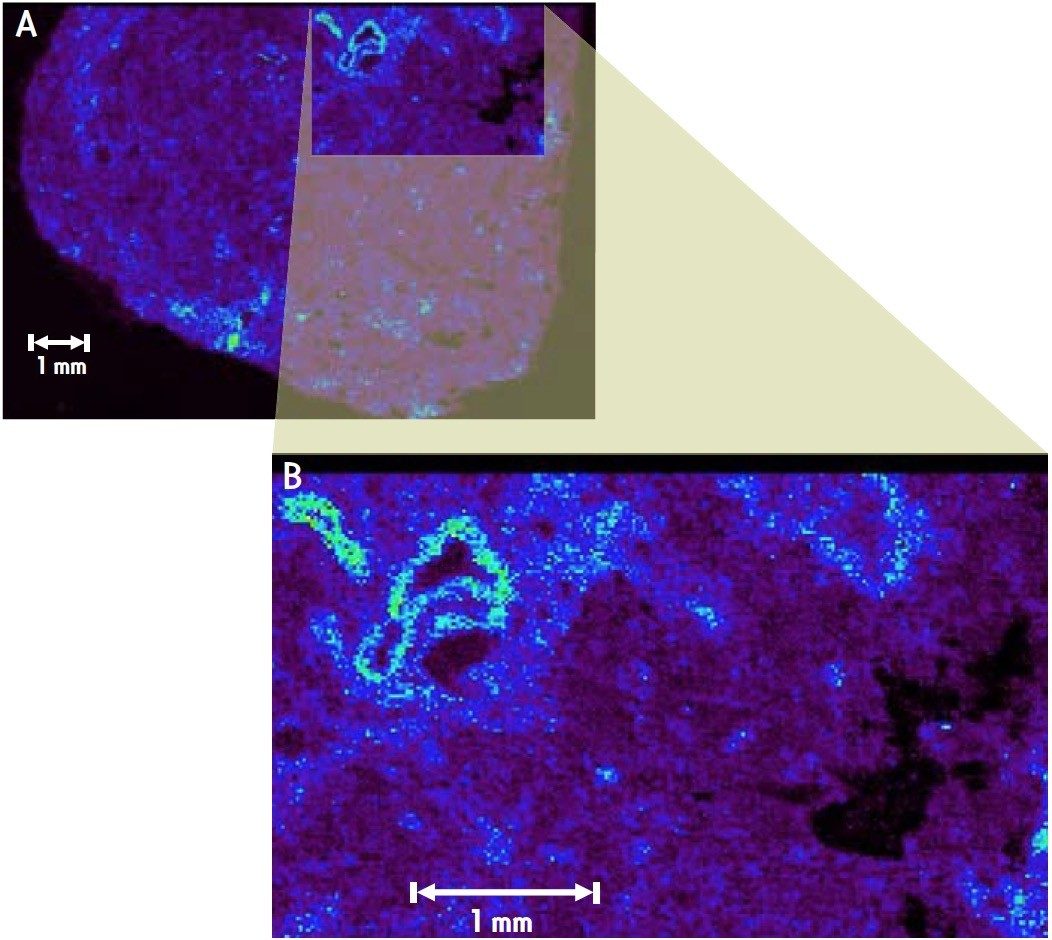
The use of a 1 kHz laser significantly reduces the time taken to generate high spatial resolution images in comparison with a 200 Hz laser. For imaging experiments on large tissue sections (> 1cm2), the use of a larger spot size accelerates the time taken to complete the imaging experiment.
The MALDI SYNAPT G2 HDMS System allows MALDI imaging experiments to be performed rapidly with the use of increased laser repetition rates, and the spatial distribution of analytes to be determined more confidently using a small laser spot profile.
The use of high efficiency ion mobility separations in imaging experiments (High Definition Imaging (HDI MALDI) can be used to more accurately and confidently image compounds across complex matrices by allowing the post ionization gasphase separation of analytes from background interferences.
720003535, May 2010