In this study, we demonstrate the capabilities of the ACQUITY UPLC System in quantifying perindopril isomers by starting from an HPLC method. The results obtained from the ACQUITY UPLC System showed better peak resolution and peak shape with shorter run times.
The UPLC method enables users to run separations using shorter columns for increased speed, and provides superior resolution and sensitivity. This leads to increased lab productivity, decreased operational costs, and improved product development.
Perindopril, or perindopril arginine, is a long-acting ACE inhibitor. ACE (angiotensin-converting enzyme) inhibitors, including peridopril, are commonly used to treat high blood pressure, hypertension, heart failure, or stable coronary artery disease. However, use of perindopril is plagued by an isomerization problem. The S-perindopril enantiomer, shown in Figure 1A, is the enantiomer responsible for ACE inhibition, whereas, the presence of R-perindopril enantiomer, shown in Figure 1B, is undesirable.
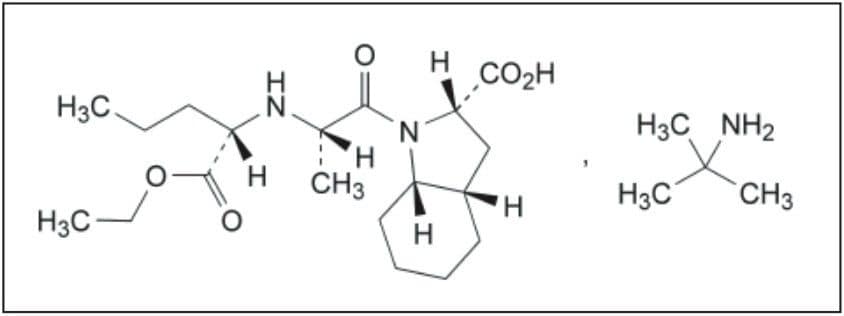
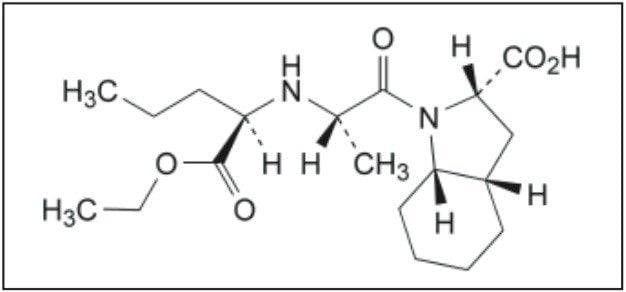
The European Pharmacopiea describes a liquid chromatography-based method for the quantification of the perindopril enantiomers. Chromatography is the most versatile and widespread technique employed in modern analytical chemistry, due to its sensitive detection methods, fast separation capabilities, and accuracy. Although HPLC has been traditionally used for liquid chromatography for several years, the Waters ACQUITY UPLC System enables analytical laboratories to deliver better quality results, compared to those obtained using conventional liquid chromatography instrumentation. Improved resolution (Rs) and sensitivity (LoQ) are often accompanied by greatly reduced runtimes, directly translating into significant commercial benefits. This combination of uncompromised data quality with speed makes ACQUITY UPLC a disruptive technology, which is destined to displace conventional liquid chromatography instrumentation in the near future. Quick method transfer and validation is the first step in this process.
A typical example, which demonstrates the benefits as well as establishes the ease of method transfer, shows determination of stereochemical purity of perindopril tertbutylamine1 described in the European Pharmacopeia. In this study, we demonstrate the capabilities of the ACQUITY UPLC System in quantifying perindopril isomers by starting from an HPLC method. The results obtained from the ACQUITY UPLC System showed better peak resolution and peak shape with shorter run times.
Perindopril tert-butylamine certified reference standard CRS was obtained from Aarti Healthcare Limited (Mumbai, India). The active pharmaceutical ingredient (API) was obtained from commercial sources. Sodium heptane sulfonate, perchloric acid, and pentenol were procured from Ranbaxy Fine Chemical (India) Ltd., while ‘BAKER ANALYZED’ HPLC solvent grade water and acetonitrile were obtained from J.T. Baker. Solutions of both CRS and API form of perindopril tert-butylamine were prepared following the protocol detailed in the European Pharmacopiea1 (henceforth referred to as EP).
|
HPLC system: |
Waters Alliance 2695 |
|
HPLC column: |
Inertsil ODS-3 C18 4.6 x 250 mm, 5 μm |
|
Detector: |
2996 Photo Diode Array Detector |
|
UPLC system: |
Waters ACQUITY UPLC (binary) |
|
UPLC column: |
Waters ACQUITY UPLC HSS C18 2.1 x 100 mm, 1.8 μm |
|
Detector: |
2996 Photo Diode Array Detector |
Empower 2 Software
The isocratic HPLC method described in the EP was verified using sodium heptane sulfonate (pH 2 adjusted with a mixture of perchloric acid and water (1:1 v/v), acetonitrile, and pentenol in the v/v ratio of 78.0:21.0/7.0:0.3. Column temperature was maintained at 50 °C while the sample compartment was maintained at 15 °C. The column elution was monitored at 215 nm. The chromatography system was equilibrated at a flow rate of 0.8 mL/min for four hours (as specified in the EP Monograph) followed by standard and sample chromatography acquisitions with an injection volume of 10 μL.
The run time for each HPLC analysis was approximately 180 min. The analysis passed EP system suitability criteria specified for this particular analysis. Having thus verified that commercially obtained API for perindopril tert-butylamine passed the EP stereochemical purity test, the method was transferred to the ACQUITY UPLC System.
The parameters for the UPLC method were quickly obtained by entering the HPLC method parameters into the ACQUITY UPLC Column Calculator v1.1. The ACQUITY UPLC HSS C18 1.8-μm 2.1 x 100 mm Column was selected by using Waters’ column selection guide software. Column selection guide software is a simple tool for finding the equivalency of the Waters or non-Waters HPLC columns with Waters ACQUITY UPLC Columns; it is available on the system console. Flow rate and injection volume recommended by the calculator were set to 0.55 mL/min and 1 μL, respectively. The column and sample compartment temperatures recommended in the EP method were maintained without change.
The pump pressure, which is 40 MPa (400 atmospheres) in HPLC, can go up to 100 MPa in UPLC. UPLC also allows for much smaller particle size, normally less than 2 μm whereas it is normally 5 μm in HPLC. Compared to conventional HPLC methods, significantly shortened run times with better resolution and peak shape are possible with UPLC.
Using the above-mentioned parameters, the final run time per sample with UPLC was reduced to approximately 20 min. The ACQUITY UPLC System method better suited the system suitability criteria, as described in the European Pharmacopiea (Table 1).
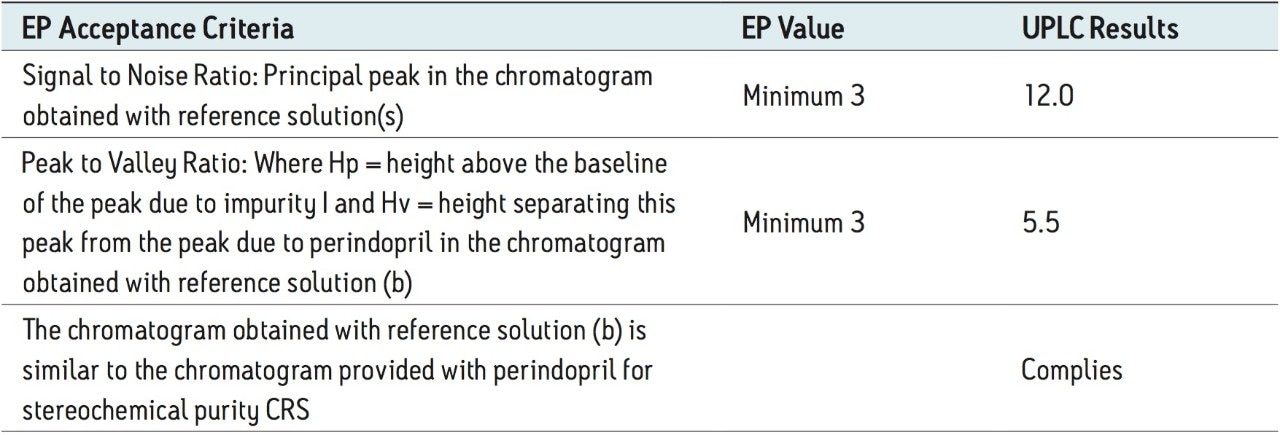
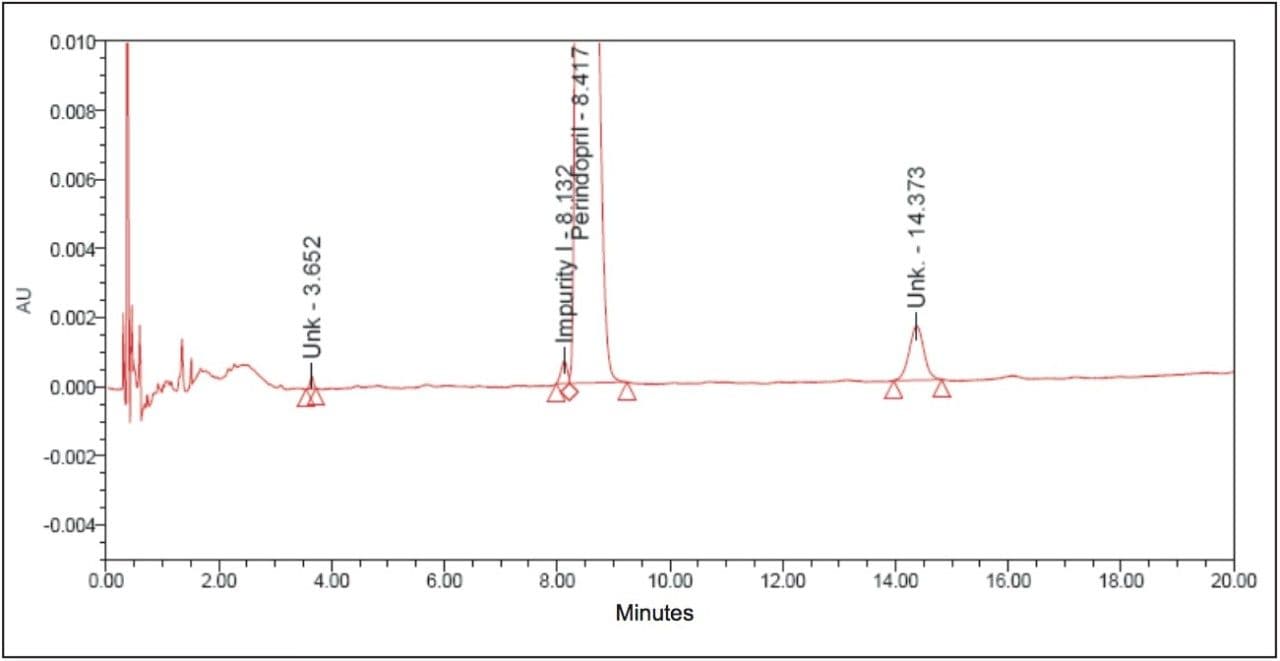
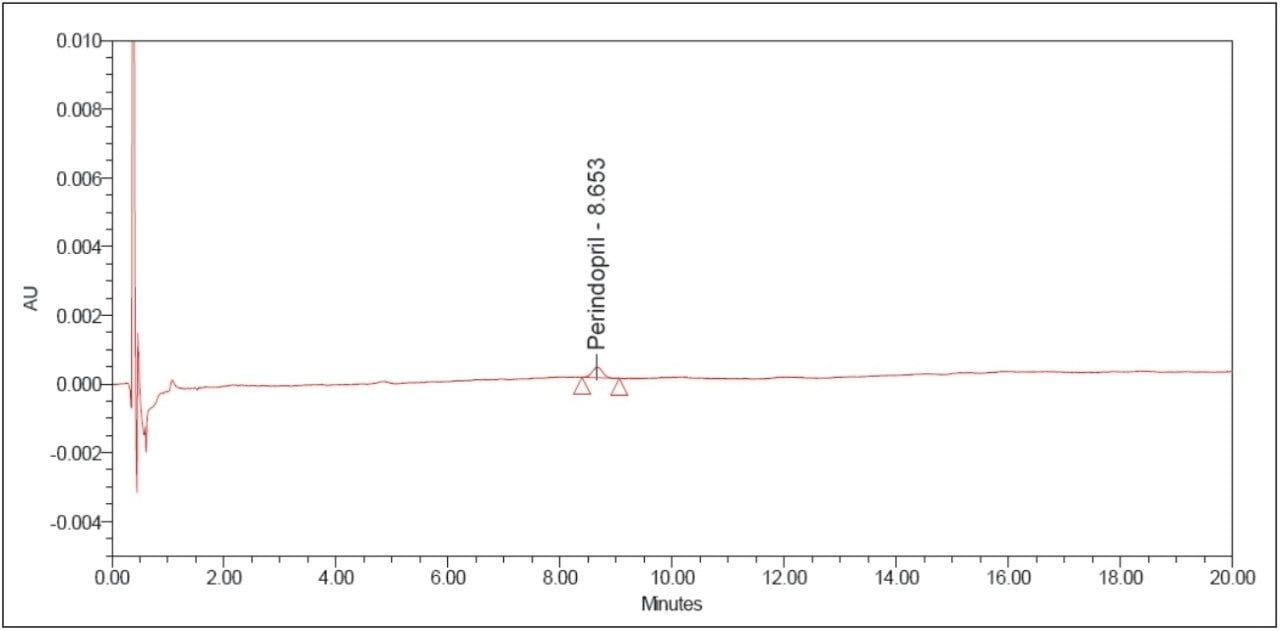
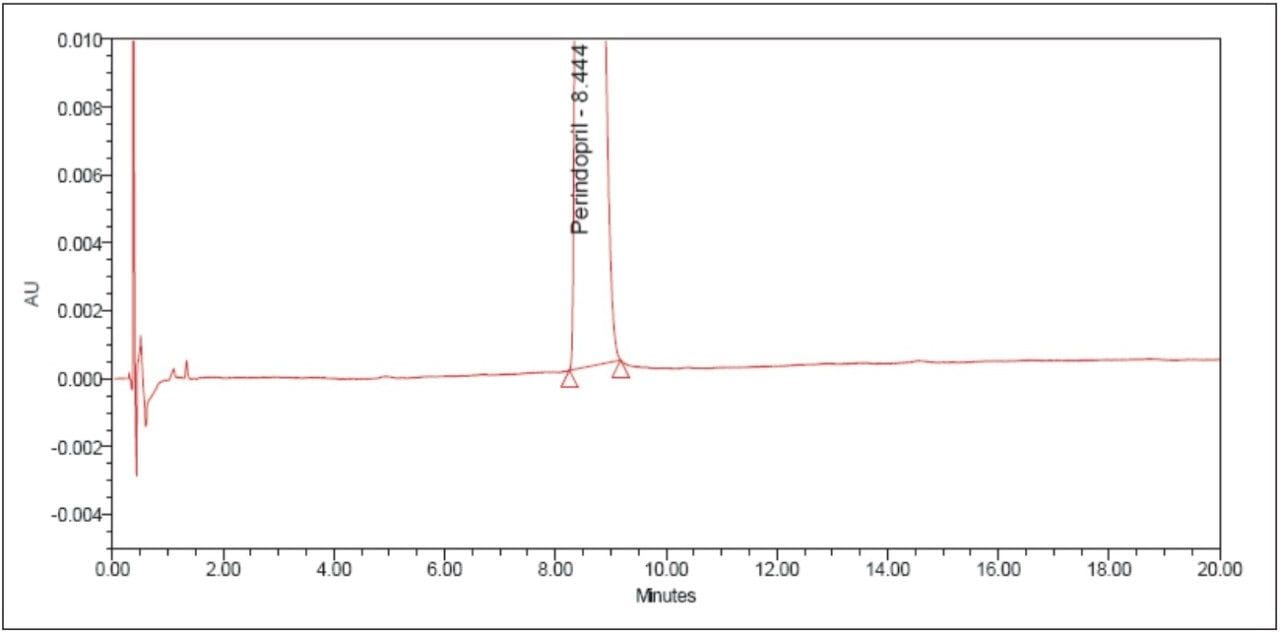
The above example briefly demonstrates the improvement of chromatographic quality and the time advantage when an HPLC method is transferred to an ACQUITY UPLC System. Better peak shape, resolution, and sensitivity were observed with UPLC, as shown in Figures 3 and 4. System suitability parameters for the UPLC method were found to be significantly better than those in EP acceptance. In addition, the analysis time on the UPLC method was reduced by a factor of 8X to 20 min from its corresponding HPLC method of 180 min.
Transfer of the legacy HPLC method to the ACQUITY UPLC System method can be quickly done using the ACQUITY UPLC Columns Calculator v1.1. Subsequently, a comprehensive set of validation experiments can be set up and run with ease using the Empower 2 Method Validation Manager.
Similar benefits have been observed for virtually all method transfers done on an ACQUITY UPLC System. This reinforces the concept that ACQUITY UPLC is a disruptive technology, which poses several benefits over the conventional HPLC technology. It is destined to displace conventional liquid chromatography instrumentation in the analytical laboratory.
720004200, January 2012