
This is an Application Brief and does not contain a detailed Experimental section.
This application brief shows nodularin and the major microcystins of concern in drinking water that can be analyzed with minimized sample preparation and increased confidence in results.
Increased sensitivity with reduced run time, minimized sample preparation and solvent consumption for microcystin analysis.
There is an increased interest in the monitoring of microcystins that are generated by blue-green algae in drinking water in order to protect the public from exposure1. EPA Method 544, for instance, monitors for six microcystins and nodularin, and utilizes solid phase extraction (SPE) and LC-MS/MS to reach the minimum reporting level of 1 µg/L.2
One major challenge in using some current methods is they involve SPE extraction of 500 mL of water that is subsequently concentrated down to 1 mL. This process is time consuming as the loading and evaporation of the extract required to meet necessary detection levels can take hours. However, with less sensitive instrumentation, this is the only way that the challenging regulatory limits can be met.
Another challenge with the current method is the use of a single MRM transition for each analyte. This makes it difficult to confirm spurious results and can lead to re-analysis and delays in reporting results which are critical to ensure the public are not at risk from exposure. Having an analytical method that is more sensitive, with additional transitions and rapid run time provides multiple advantages in the targeted analysis of microcystins.
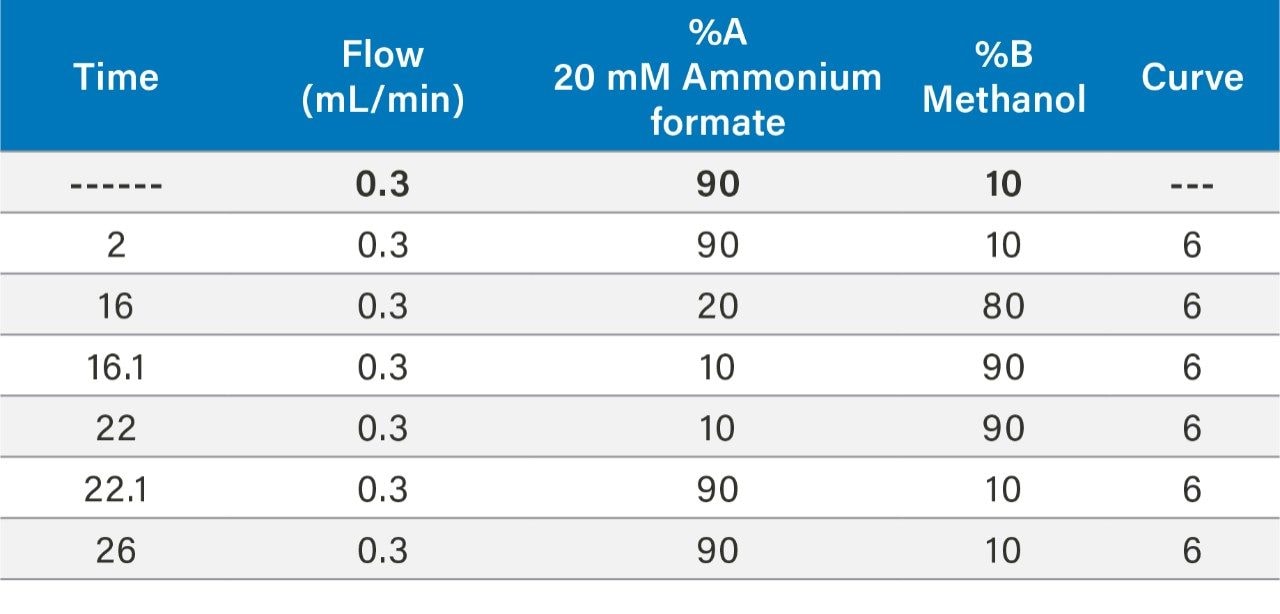
In this work, the current EPA Method 544 was used as a starting point for method development. A Waters UHPLC column and the Waters Xevo TQ-S micro were used for this investigation. The CORTECS C8 90Å, 2.7 µm, 2.1 mm x 100 mm Column (P/N 186008351) was used with a VanGuard C8 90Å, 2.7 µm, 2.1 mm x 5 mm Cartridge (P/N 186008421) and holder (P/N 186007949) for the analysis. Chromatography was further optimized to improve separation between near eluting analytes. Table 1 shows the final chromatographic conditions utilized for this analysis. Figure 1 shows the separation defined in EPA Method 544 while Figure 2 shows the separation on the CORTECS Column. The method showed comparable separation to the current column used in EPA Method 544 and detection of the seven compounds of interest.
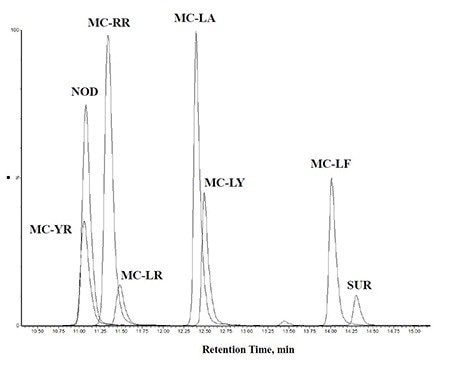
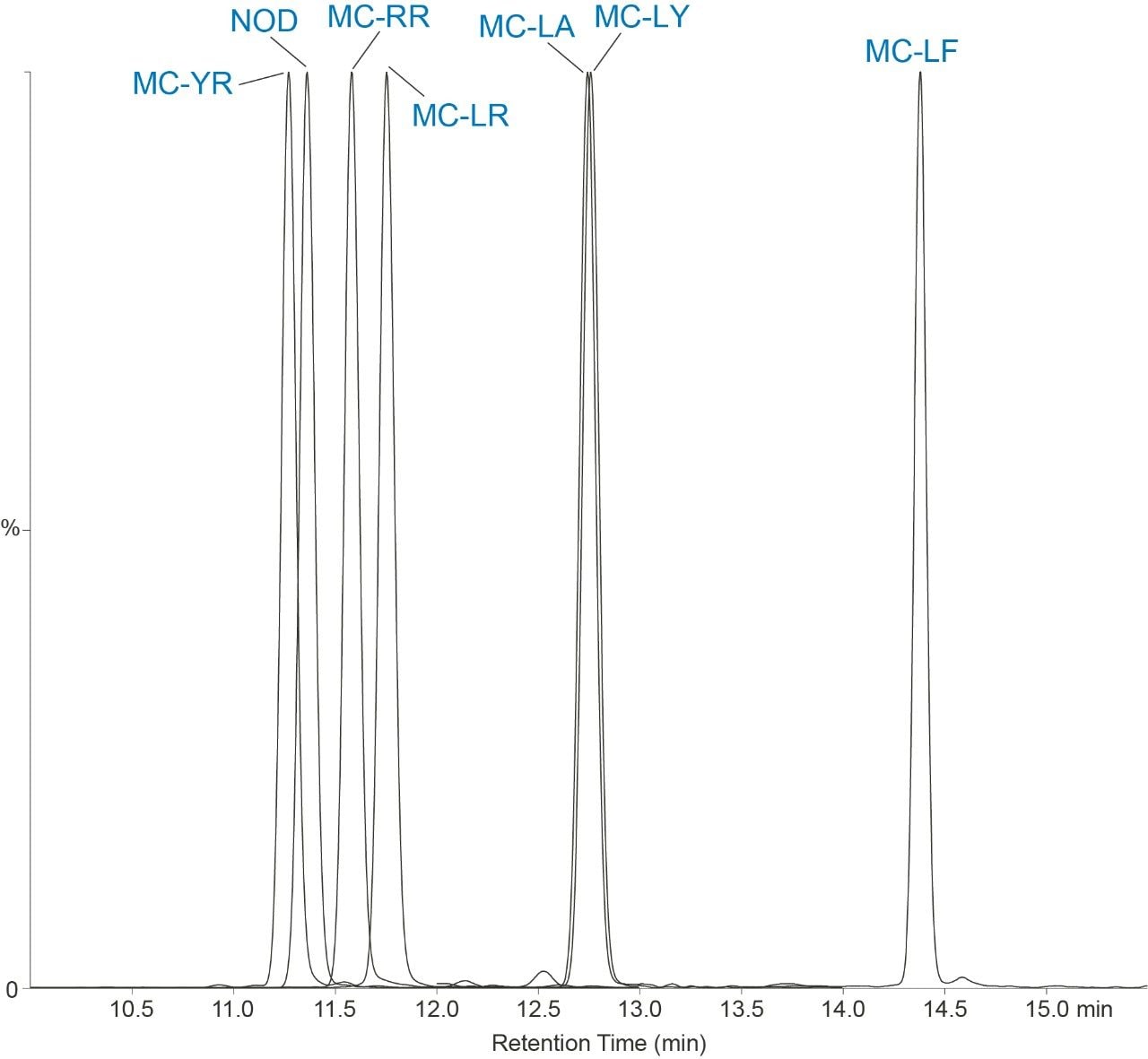
The seven compounds of interest were optimized on the Xevo TQ-S micro. An additional MRM transition was added for each compound. This allowed for further confirmation of the presence of the compound and verification of not only an additional transition but the ion ratios between the two transitions.
As the sensitivity of the Xevo TQ-S micro was excellent, no SPE or pre-concentration of drinking water was required for any of the work. While EPA Method 544 does not allow for the exclusion of SPE, this work does demonstrate that current generation tandem quads are able to meet the method’s challenging detection requirements even without the enrichment provided by SPE sample preparation.
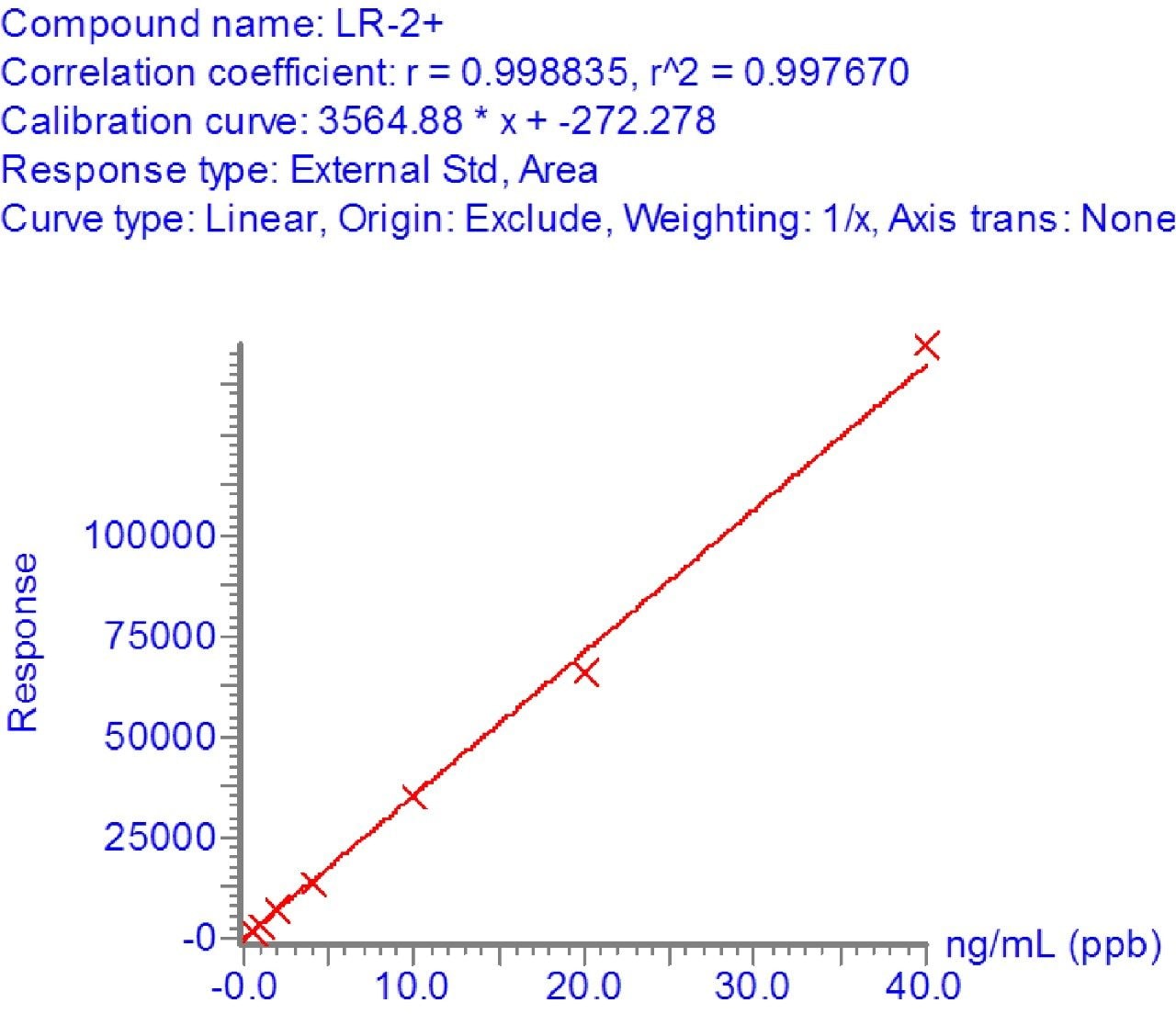
In order to assess the sensitivity of the method, a calibration curve was made of microcystin LR, YR, and RR compounds between 0.5 and 40 ppb in drinking water. The linearity and limit of detection were excellent as indicated by the R2 values of >0.99 and %RSDs of less than 15%. Figure 3 shows the linearity of microcystin LR and Figure 4 shows the detection of microcystin LR 0.5 ppb.

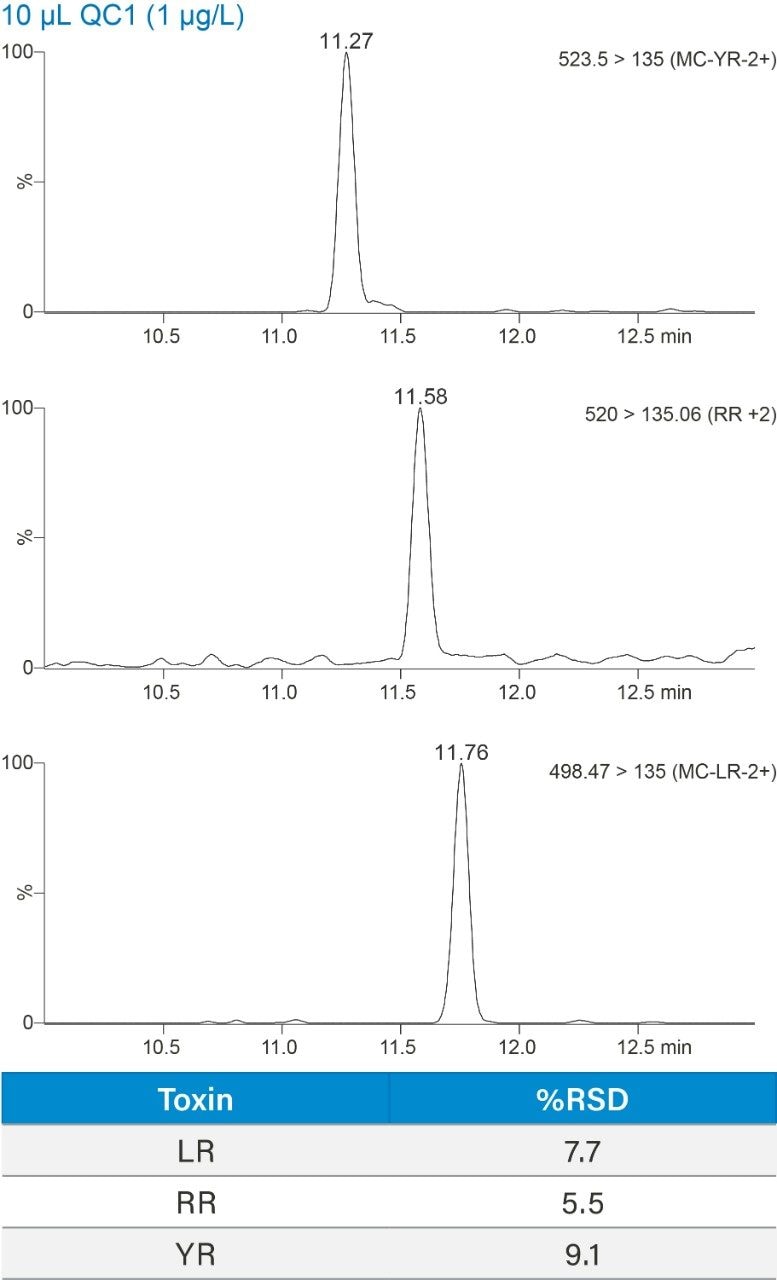
Finally, in order to ensure the method was reproducible, three example microcystins were spiked into a drinking water sample at 1 µg/L and injected 5 times. The % RSDs under 10% for the replicates fall within the requirements described in EPA Method 544.
The use of the CORTECS C8 Column produces equivalent chromatographic separation within a shorter run time for the nodularin and the six microcystins investigated. Although EPA Method 544 does not allow for the exclusion of SPE, the increased sensitivity of the Xevo TQ-S micro allows the user to potentially eliminate SPE or use less water to concentrate while still meeting the challenging detection limit requirements for current analytical methods. The addition of a confirmatory MRM transition for each compound also ensures that the compound is accurately detected and reported.
720006390, September 2018