Exceptional Carryover Performance of the Arc HPLC System for Chlorhexidine Sample
Abstract
Chlorhexidine is a broad-spectrum antimicrobial biguanide used as a topical antiseptic and in dental practice for the treatment of inflammatory dental conditions caused by microorganisms. It is one of the most common skin and mucous membrane antiseptic agents in use today.
It is typically viscous in nature and, therefore, very difficult to remove from injector surfaces and is known to give injector carryover in HPLC systems, which may result in out-of-specification values and may eventually lead to batch failure. We have performed the USP monograph method of organic impurities for chlorhexidine gluconate using an Arc HPLC System and the carryover is not detected in Arc HPLC when compared to competitor HPLC systems. The experiment confirms that the Arc HPLC System can minimize the injector carryover issue for chlorhexidine.

Benefits
- Carryover is a major challenge for chromatographers in many laboratories and impacts the accuracy of results and quality of data being produced, reduces sample throughput, and increases assay complexity
- The Arc HPLC System with flow-through needle design can successfully reduce the carryover of an analyte
- User-configurable wash settings provide capability to address injector carryover issue
Introduction
“Carryover" is defined as “sample left over from a previous injection that may interfere or co-elute with analytes of interest, often interfering with accurate quantitation.” Carryover has always been a problem in HPLC systems, but its significance increases with today’s sensitive detection methods. Carryover is detected by the unexpected presence of small peaks when a blank sample is injected following the injection of analyte samples. It can also be detected by unexpectedly poor injection-to-injection precision following multiple sample injections. There are several factors that can influence injector carryover, including analyte chemical nature, column chemistry, and HPLC system injector design.
The Arc HPLC System allows you to easily replicate and improve the performance of existing LC methods without comprising the data quality. It also provides the advantages of low analyte carryover, high injector precision, and high back pressure tolerance to enhance existing HPLC methods and improve the efficiency and performance of your analysis.
The experiment conducted here was to resolve the carryover issue of chlorhexidine gluconate.
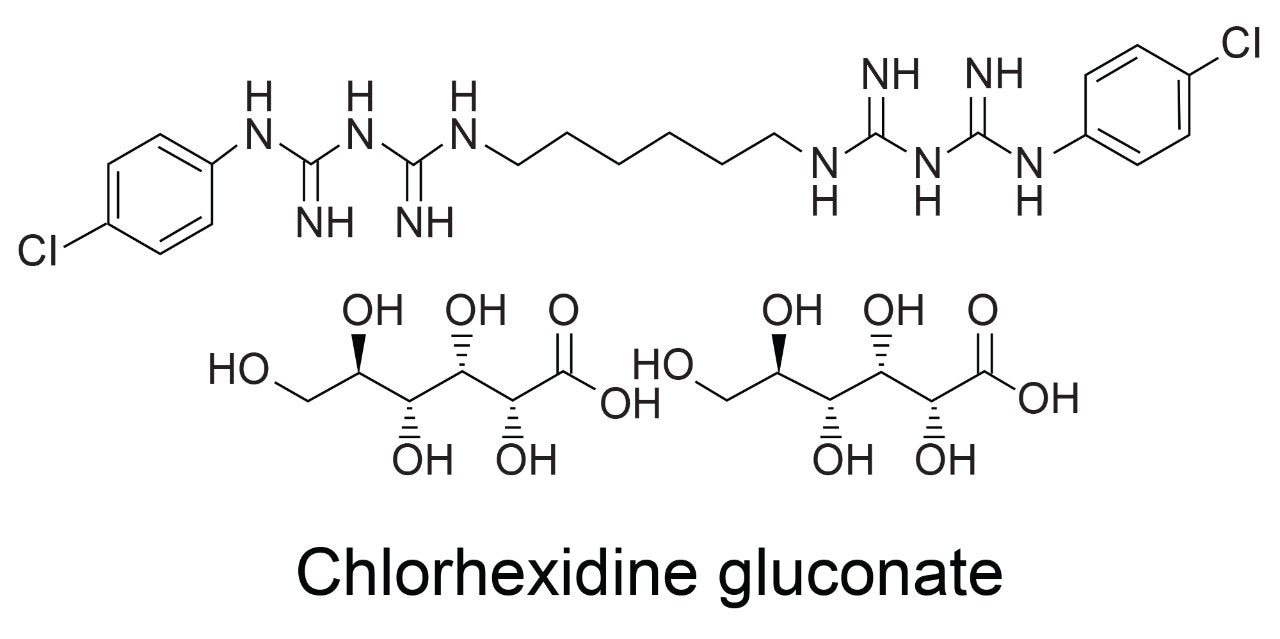
Experimental
Analytical Method Conditions
|
System: |
Arc HPLC System with with 2998 PDA Detector |
|
Column: |
XSelect HSS C18, 3.5 µm |
|
Column temp.: |
30 °C |
|
Flow rate: |
1.0 mL/min |
|
Mobile phase A: |
0.1% TFA in water:0.1% TFA in ACN (80:20) |
|
Mobile phase B: |
0.1% TFA in ACN:0.1% TFA in water (90:10) |
|
Detection: |
254 nm |
|
Injection volume: |
10 µL |
|
Purge solvent: |
Water:acetonitrile (9:1) |
|
Needle wash solvent: |
Water:acetonitrile (9:1) |
|
Run time: |
65 min |
|
Sampler temp.: |
8 °C |
|
Diluent: |
Mobile phase A |
|
Test sample: |
Diluted 100 times of 20% aqueous chlorhexidine gluconate sample |
|
Dilute sample: |
Diluted 100 times of test sample with diluent |
Table 1. Analytical method conditions.
Gradient
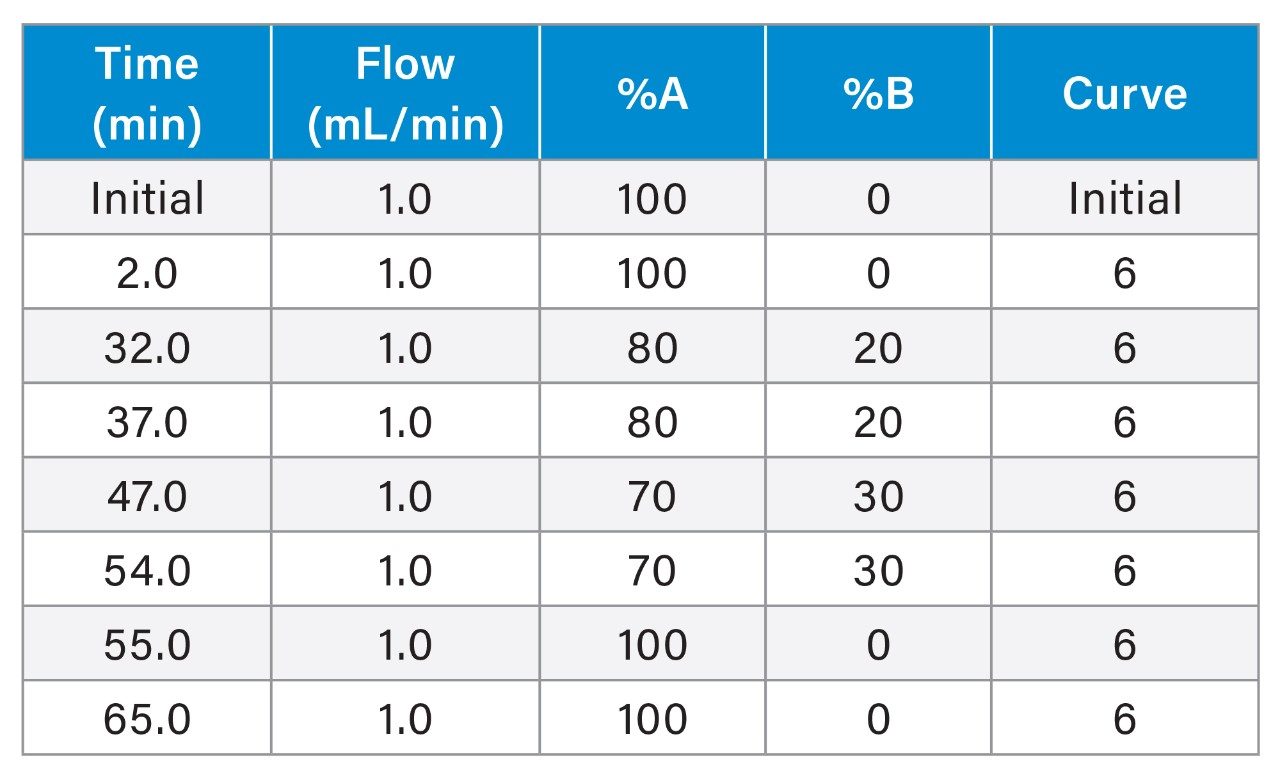
The experiment was designed to investigate the injector carryover in post-blank chromatogram of chlorhexidine gluconate sample. Injected two sets of sample sequence in the following pattern.
Sample Set
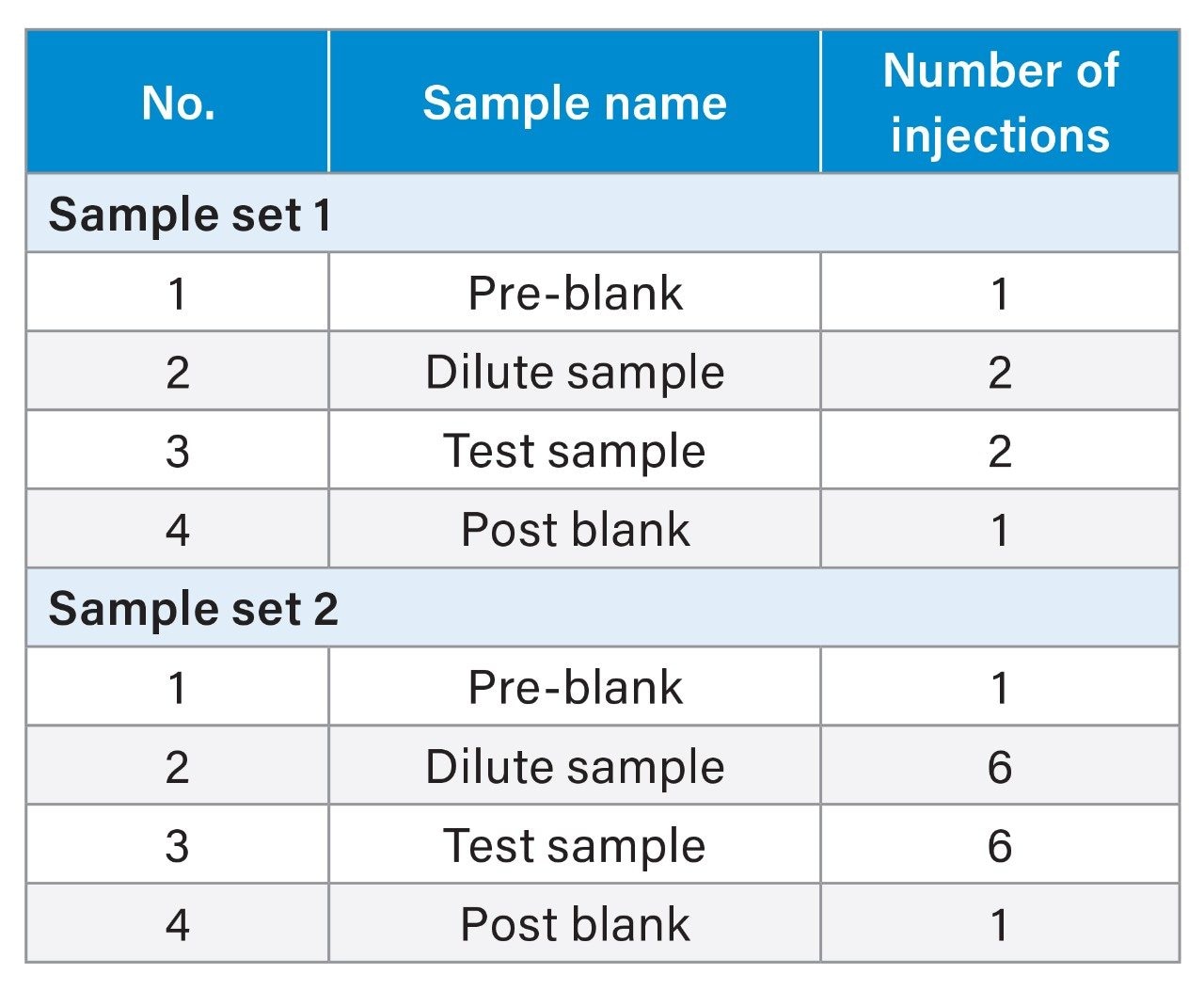
The method adopted on the Arc HPLC System is a USP monograph organic impurities method for chlorhexidine and the chromatographic conditions were replicated in the competitor HPLC system. For sample set 1, the %carryover observed in the competitor HPLC system is 0.0015%, whereas in the Arc HPLC System carryover is not detected. For sample set 2, the %carryover increased from 0.0015% to 0.0027% in the competitor HPLC system, as the number of test sample injections are sequenced in the run, but in the Arc HPLC System carryover remains undetected.
Results and Discussion
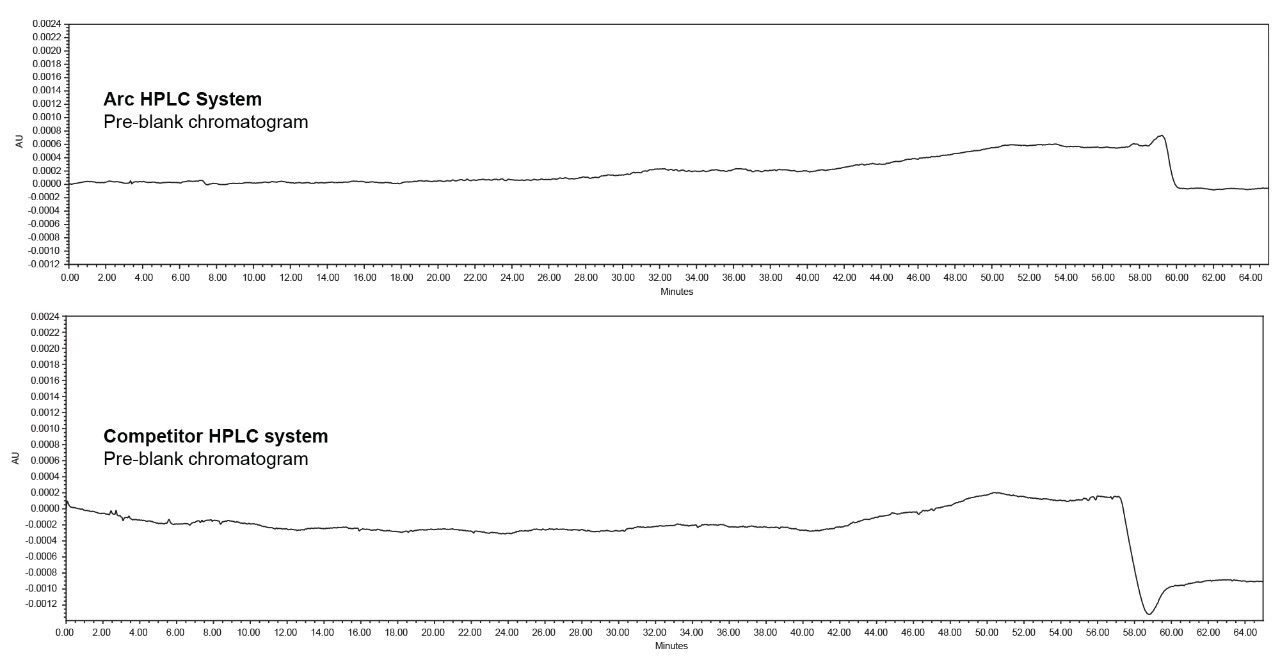
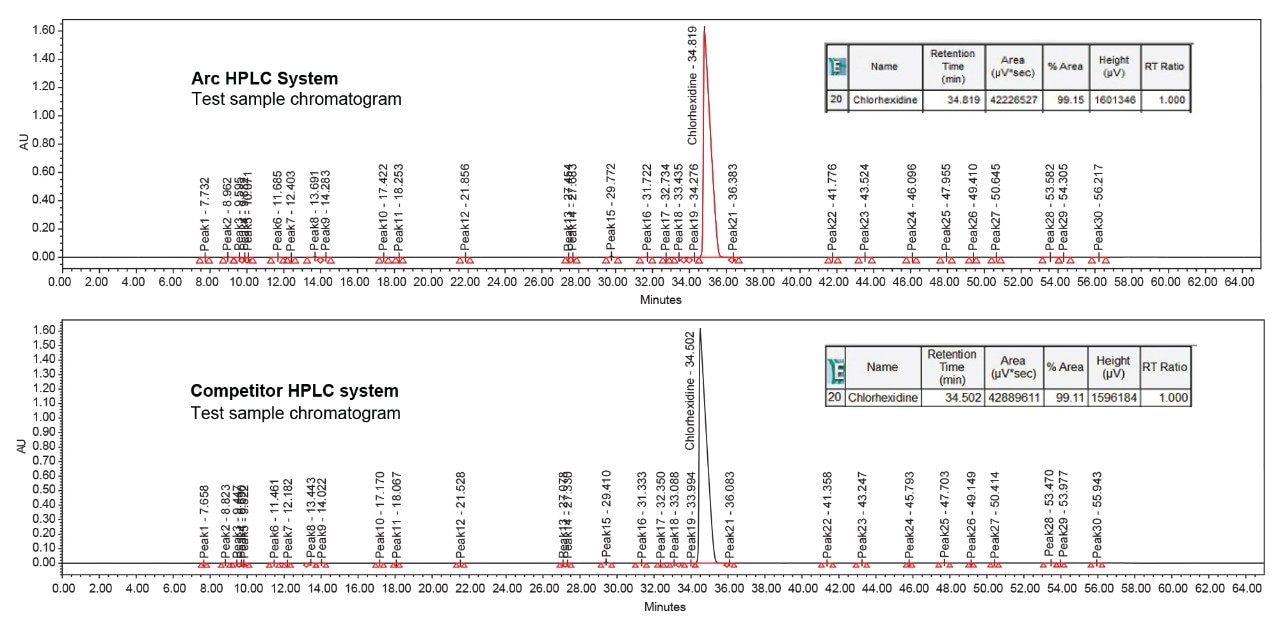
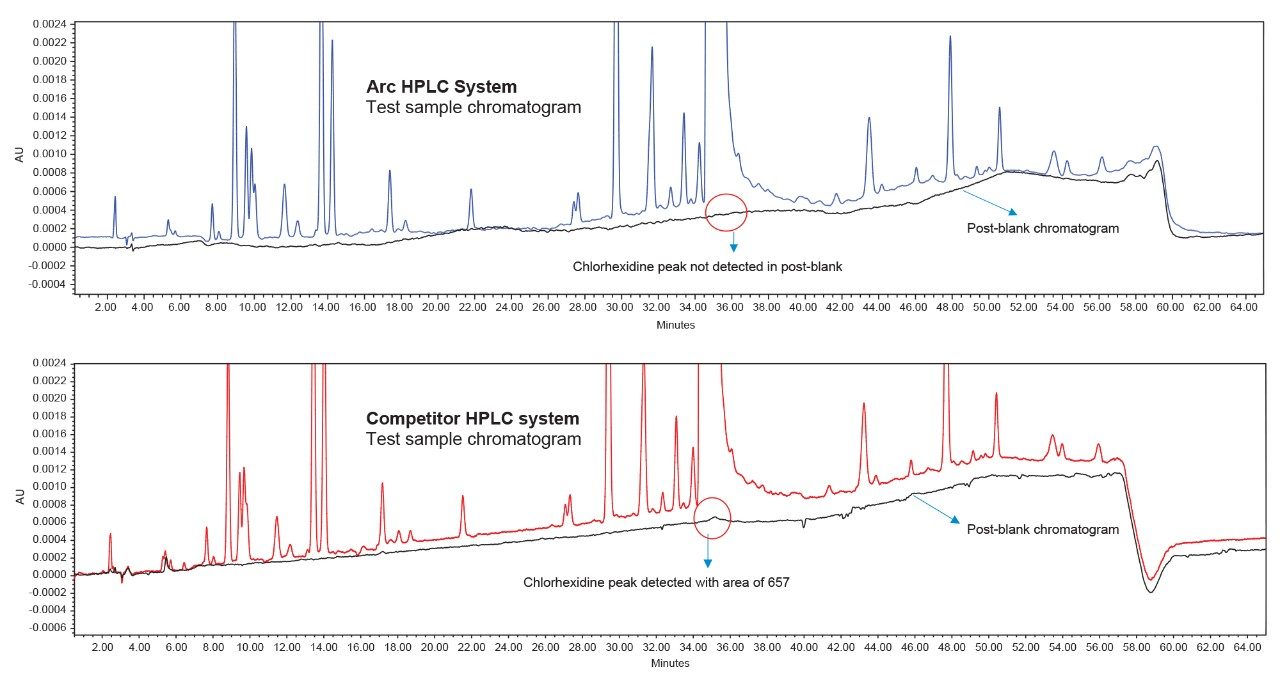
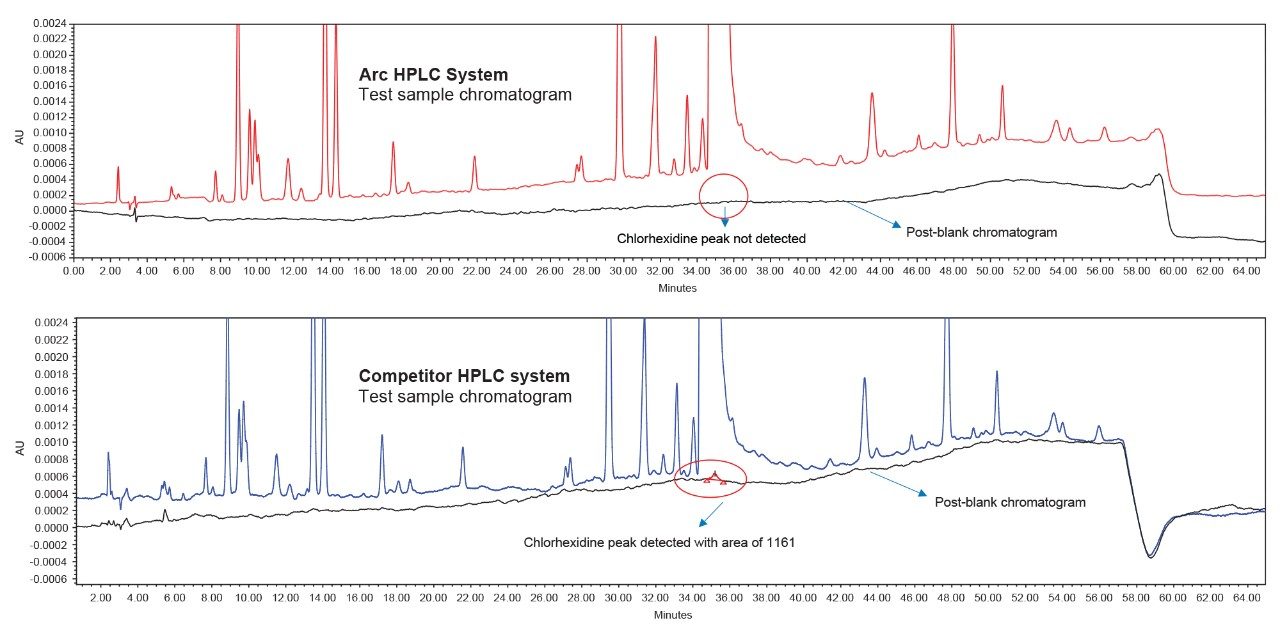
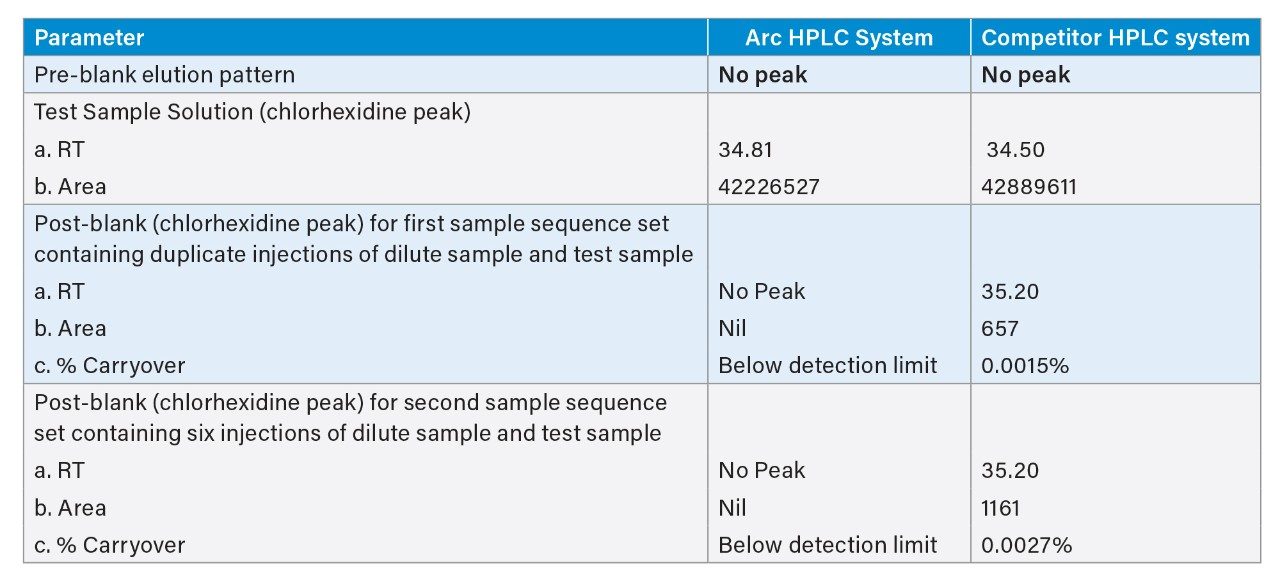
The advanced flow-through needle design of the Arc HPLC System helps to minimize injector carryover, as the interior of the sample injection needle is continuously washed during the run. This allows you to enhance injector performance without redeveloping your method. In the competitor HPLC system, carryover was observed in the first post-blank injection and in the diluted sample solution, which impacts the impurities results. In addition to injector carryover, analyte chemical nature and column chemistry are also possible causes of carryover. While performing the USP monograph method of organic impurities using chlorhexidine gluconate and the same column chemistry, exceptional carryover performance was observed on the Arc HPLC System.
Conclusion
- The Arc HPLC System shows exceptional carryover performance for the chlorhexidine gluconate sample.
- Injected multiple injections (~70 injections) of test sample solution and diluted sample over four days and observed the carryover is below detection limit.
- The experiment confirms that the Arc HPLC System resolves the injector carryover issue for chlorhexidine gluconate samples.
References
- Dlugasch, A.; Simeone, J.; McConville, P. Alliance Carryover Performance Part 1: Carryover Improvement Achieved Through Instrument Design Changes for the Alliance HPLC System. Waters Application Note, 720006386EN, 2018.
- Bheeshmacharyulu S; Boyidi, T.; Pullancheri, D.; Wagh, P. Successful Achievement of Ultra Low Injector Carryover of Benzyl Alcohol Using Arc HPLC. Waters Application Note, 720007076EN, 2020.
Featured Products
720007420, November 2021