Instrument Considerations for Successful Adaptation of Amino Acid Analysis Methods Which Utilize Pre-Column Derivatization From an ACQUITY UPLC to an ACQUITY UPLC H-Class PLUS Binary System
Abstract
Amino acid analysis (AAA) by liquid chromatography has its own unique challenges due to the chemical and physical properties of the analytes. Since most amino acids have no chromophore, a derivatization technique, such as AccQ•Tag, is often used for analysis by UV. In addition, the varied chemical properties make separation of a wide range of amino acids challenging. With this in mind, the AccQ•Tag Solution was released in 2007 using the Waters ACQUITY UPLC System to provide a complete solution for amino acids in a variety of matrices. However, with improvements in instrumentation, the migration of the analysis of amino acids to newer systems was needed.
In the following study, the methods used for amino acid analysis using AccQ•Tag derivatization will be migrated from the ACQUITY UPLC to the ACQUITY UPLC H-Class PLUS Binary System. It is critical to understand how instrument design differences can impact this process, while preserving all critical performance characteristics, which can include peak shape, resolution, linearity, limits of detection/quantification, and intra/interday precision to name a few. In this application note, we will show successful method adaptation for multiple amino acid application areas, including those amino acids found in protein hydrolysate, cell culture, food and feed, and alkylated cysteines samples, from the ACQUITY UPLC to the ACQUITY UPLC H-Class PLUS Binary System. The critical performance characteristics have been maintained after method adaptation. Additionally, quantitative analysis of taurine in multiple energy drinks yielded nearly identical results, further proof that the method adaptation has been successful.
Benefits
- AccQ•Tag Ultra Chemistry Kit including column, standards and reagents, and eluents for fast, reliable, and reproducible amino acid derivatization, separation, and quantification
- ACQUITY UPLC H-Class PLUS Binary System provides greater precision for challenging gradients and increased speed for high-throughput analysis
- Increased lab productivity and flexibility demonstrated by adaptation of established methods to newer technology
- All critical performance characteristics are maintained, including peak shape, resolution, linearity, limit of quantification, inter/intraday precision after method adaptation
Introduction
Amino acid analysis (AAA) by liquid chromatography has its own unique challenges due to the chemical and physical properties of the analytes. Additionally, with the current state of the pharmaceutical industry, it is likely that developed LC methods will be run on a variety of different hardware platforms during the lifetime of the method. This may be an artifact of the global nature of the industry, where methods are commonly run in multiple labs, often across the globe, or due to evolution of hardware which includes the development of newer technologies and the obsolescence of older technology. It is good practice to evaluate multiple hardware platforms during method development to understand the impact of instrument differences, such as system volume and dispersion, column heating, and injector design to name a few.
In this work, a legacy method developed on the ACQUITY UPLC will be migrated to a newer LC platform, the ACQUITY UPLC H-Class PLUS Binary System, which includes some significant design differences. While both systems use binary high-pressure solvent delivery, the injector design and column heating are significantly different between the two systems. The classic ACQUITY UPLC utilizes a fixed loop injector and passive pre-heating for the column, whereas the ACQUITY UPLC H-Class PLUS Binary uses a flow-through needle injector and active solvent pre-heating. Because of the inherent design differences, some method modifications were required to maintain the critical performance attributes when migrating from the original ACQUITY UPLC to the ACQUITY UPLC H-Class PLUS Binary System. The final methods yielded nearly identical results in terms of peak shape, resolution, linearity, limits of detection/quantification, inter/intraday precision, and quantitative analysis of unknowns.
Experimental
Sample Description
For the amino acid hydrolysate quantitative method, calibration standards were prepared from Waters Amino Acid Standard (p/n: WAT088122) using norvaline (p/n: 186009301) as the internal standard and 0.1 N HCl as the diluent. The internal standard stock was prepared at 2500 µM in 0.1 N HCl. The final concentration of the calibrants were 1, 5, 10, 20, 50, 100, 200, and 500 μM for all amino acids (except cysteine which was 0.5, 2.5, 5, 10, 25, 50, 100, and 250 μM) and 250 µM for norvaline (internal standard). The precision sample was prepared at 500 µM (250 µM cysteine). Norvaline was kept constant at 250 µM for all standards and precision samples.
For intra/interday precision analysis, 3 replicates were derivatized, then pooled and vortexed. The sample was then subdivided into 3 equivalent aliquots and stored in the autosampler at 20 °C until analysis. Six injections from one vial was used for each precision day, for a total of 3 days (1 sample each day) x 6 injections = 18 total injections.
For the alkylated cysteine analysis, stocks of carboxymethylcysteine (CM) and pyridethylcysteine (PE) were prepared in 0.1 N HCl. The amino acid hydrolysate standard was spiked with CM, PE, and norvaline to yield a final concentration of 500 µM for all amino acids (except cysteine which was 250 µM).
The Amino Acid Cell Culture Standard (p/n: 186009300) and the Amino Acid Food and Feed Standard (p/n: 186009299) were prepared following the Amino Acid Standard Kits Care and Use Manual1 to a concentration of 500 µM (250 µM for cysteine) for all amino acids including norvaline.
Unknown samples: Two energy drink samples were diluted 1:10, 1:100, and 1:200 using 0.1 N HCl prior to derivatization and spiked with norvaline at a final concentration of 250 µM.
Derivatization of all standards and samples followed the procedure given in the Amino Acid Standard Kits Care and Use Manual.1 Eluent A1/A2 and Eluent B were prepared as directed in the UPLC Amino Acid Analysis Solution Guide.
Final LC Conditions
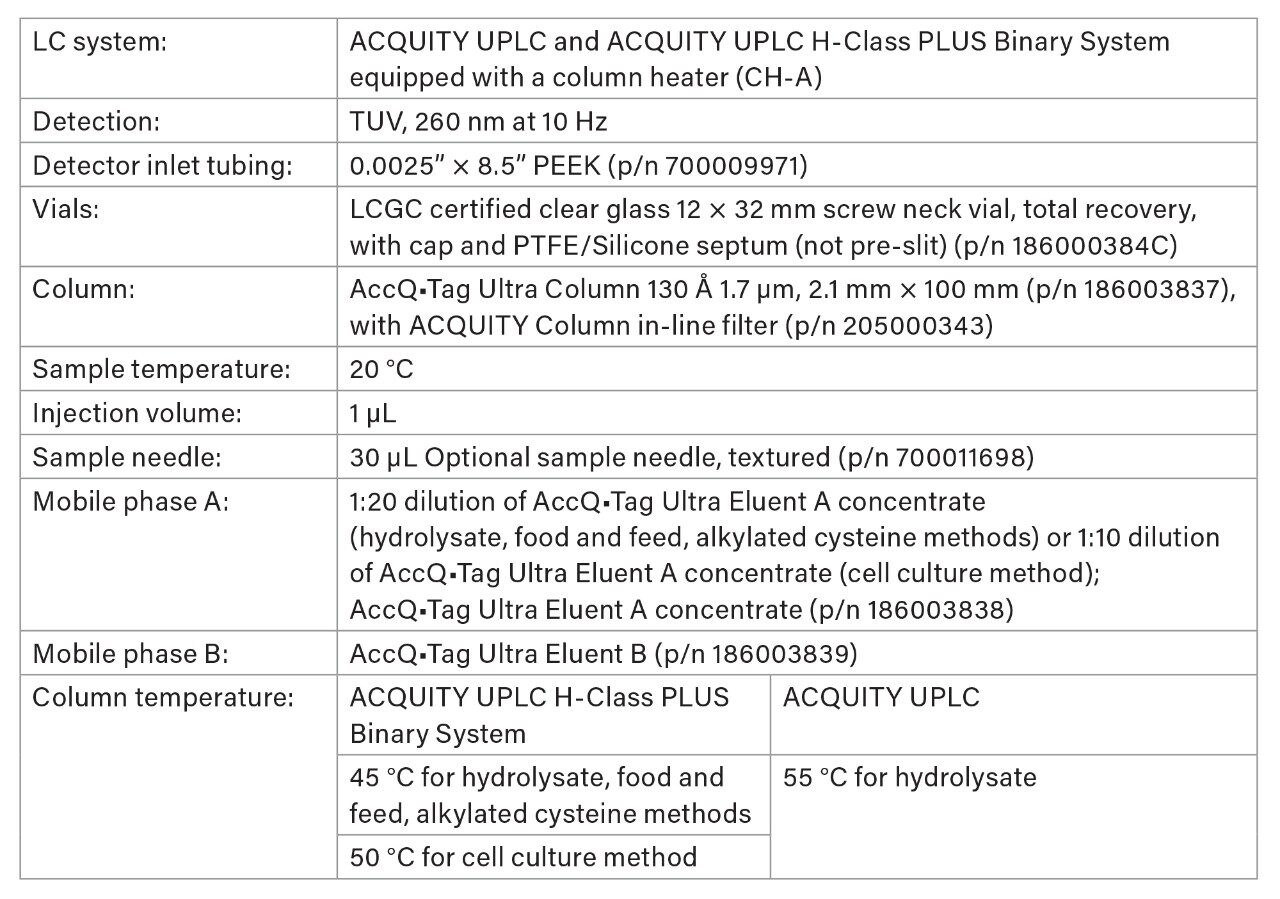
Gradient
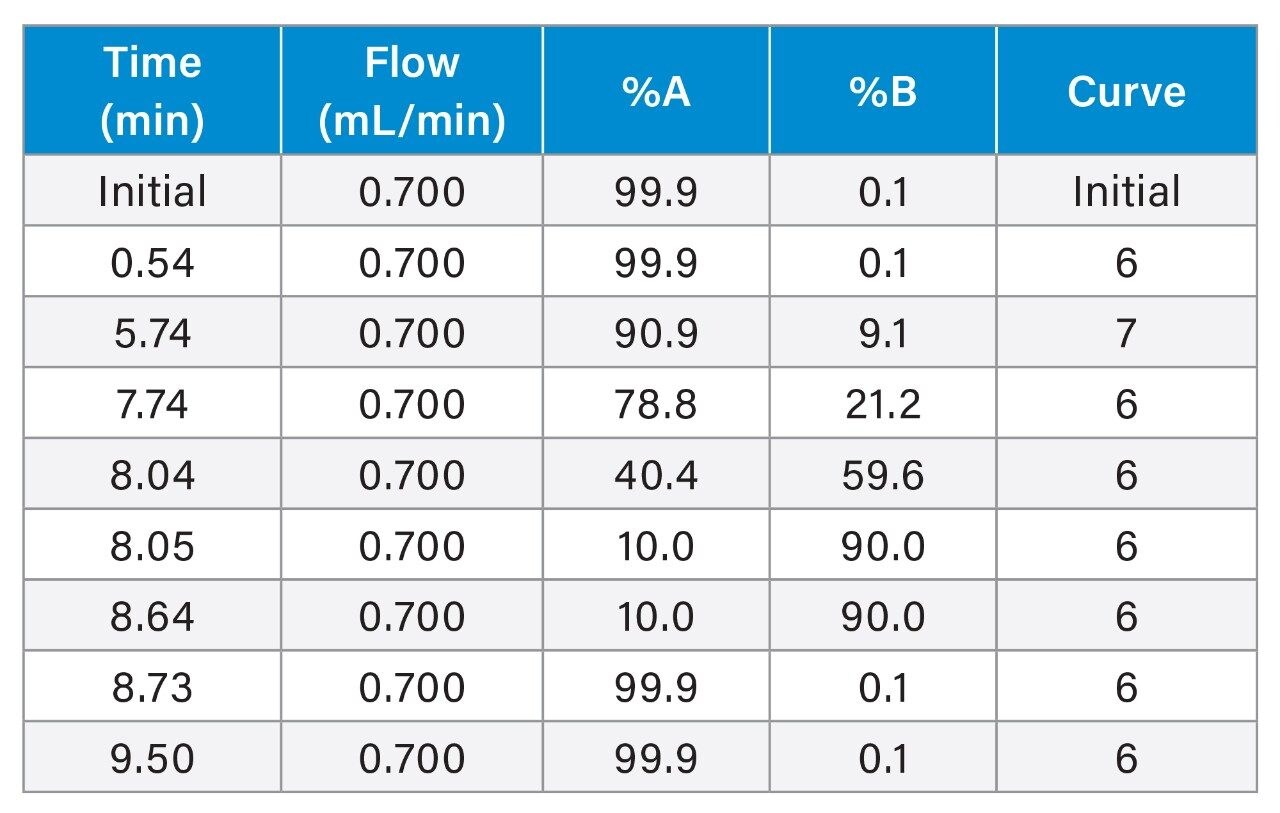
Data Management
|
Chromatography software: |
Empower 3, FR 3 |
Results and Discussion
The original Waters UPLC Amino Acid Analysis (AAA) Solution, in conjunction with Waters AccQ•Tag Ultra chemistries for amino acid analysis, was developed to analyze protein and peptide hydrolysates (for identification and characterization), cell culture media, and the nutritional composition of foods and feeds, with each group containing different or additional amino acids. These methods were developed in 2007 on the then recently developed and released ACQUITY UPLC System. In the past 14+ years, many innovations in LC hardware have been made. It is critical for both current users, as well scientists new to amino acid analysis, that we provide guidance for running these analyses on newer and/or state of the art LC hardware. The methods supplied as part of the Amino Acid Analysis Solution were developed to provide symmetrical peak shapes and adequate resolution of all peaks, and for use in quantitative analysis. It was critical to maintain all these method attributes after adaptation to the ACQUITY UPLC H-Class PLUS Binary System.
Adaptation of Method Parameters to Optimize Chromatography
Due to design and hardware differences between the systems, we anticipated that some method parameters would require adjustment to maintain the chromatographic performance when the method was moved from the ACQUITY UPLC to the ACQUITY UPLC H-Class PLUS Binary System. Initial method translation and adjustment was performed using the hydrolysate standard, then extended to the methods used for cell culture, food and feed, and alkylated cysteines analyses. Transcribing the method as written onto the ACQUITY UPLC H-Class PLUS Binary System yielded good separation of all 17 amino acids, however the earliest eluting peak, histidine, showed peak fronting due to strong solvent effects. This is due to the sample containing 20% organic from the derivatization reagent, but the initial starting conditions requiring 0.1% organic to adequately separate all early eluting peaks. It should be noted that the use of the pre-column in-line filter not only helps to protect the column by filtering out particles, but also adds additional volume which helps to mitigate strong solvent effects. To combat the strong solvent effects, multiple adjustments were made. First, the standard 15 µL needle was replaced with the optional 30 µL needle, which has a larger ID. The injection volume was maintained at 1 µL, and with the larger needle, the peak shape was improved (Figure 1A). The peak asymmetry at 4.4% was 0.50 with the standard 15 µL needle and was improved to 0.66 when the 30 µL needle was used. Additionally, area precision for a 1 µL injection was evaluated using both needles, and there was no discernible difference.
Another factor impacting the histidine peak shape is the composition of needle wash used. The needle wash is used to wash the exterior of the needle while the interior of the needle is flushed with the programmed gradient. After the needle is washed, small residual amounts of the wash solvent can remain on the needle and then get injected along with the sample of interest on the following injection. Because the injection volume used is only 1 µL, even a small amount of residual wash solvent can be enough to impact the peak shape if the wash solvent contains a high organic composition (Figure 1B). Based on the experimental results, the recommendation is to use a needle wash that is 95% aqueous and 5% acetonitrile. Carryover tests were done to ensure that the recommended needle wash composition would not result in observed carryover. No carryover was observed in an injected blank following injection of the highest calibration standard using the 5% acetonitrile wash.
Although the increased needle size and modified needle wash composition improved the histidine peak shape, the largest improvement in peak shape came when the column temperature was decreased (Figure 1C). As the temperature is decreased, the peak symmetry of histidine improves from an asymmetry value (at 4.4% peak height) of 0.51 at 55 °C to 0.76 at 45 °C. However, as the temperature is decreased, the resolution between cysteine and lysine decreases from 2.55 at 55 C to 1.70 at 45 °C. Given these two behaviors, 45 °C was chosen as the final column temperature because it was a balance between the improvement in histidine peak shape versus the decrease in resolution for cysteine-lysine.
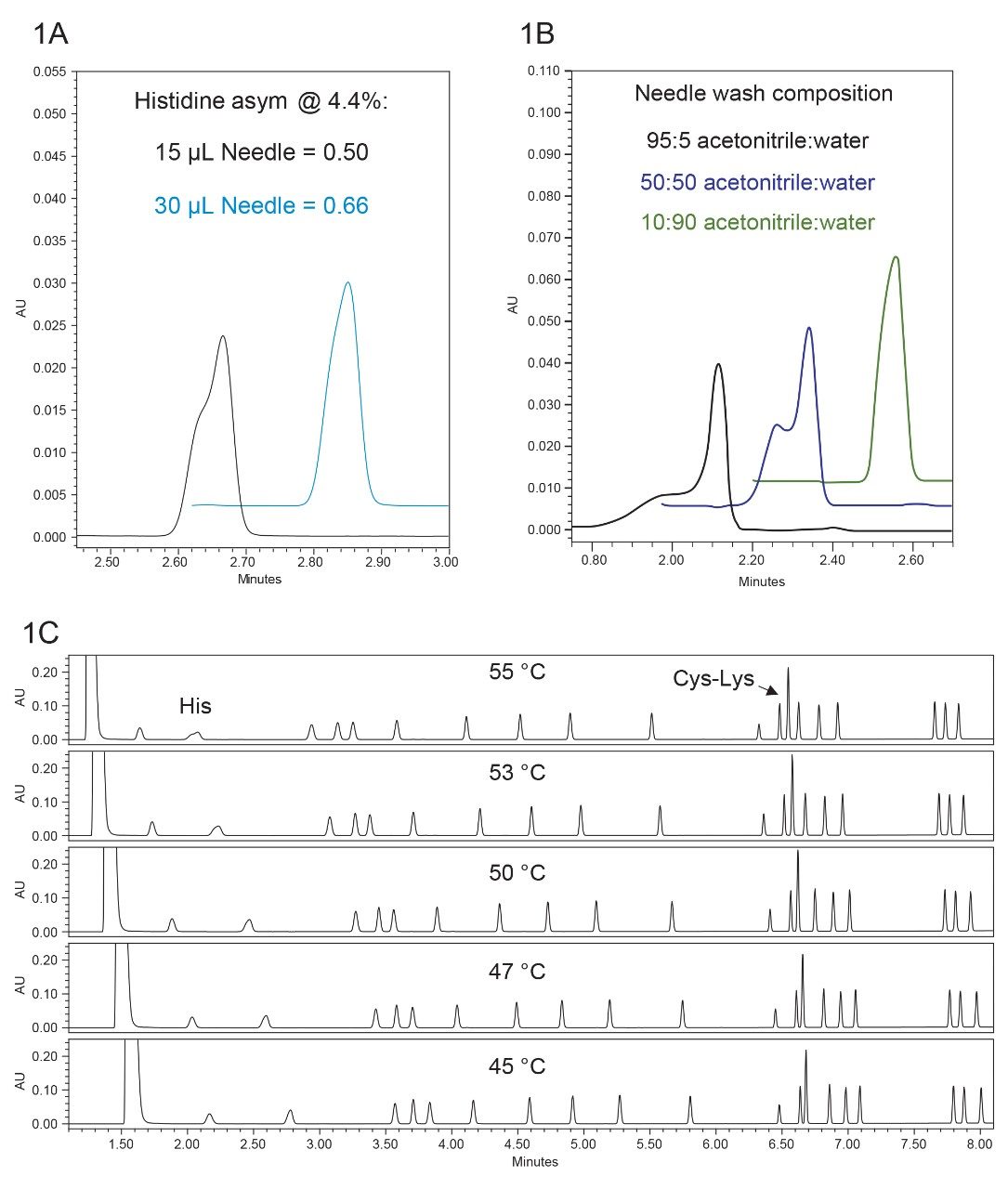
In summary, for the hydrolysate method transfer, the final instrument and method adjustments used on the ACQUITY UPLC H-Class PLUS Binary System includes use of the optional 30 µL needle, a needle wash composition of 95:5 water:acetonitrile, and a column temperature of 45 °C. Example chromatograms for a 500 µM Amino Acid Standard obtained on the ACQUITY UPLC using the original method and on the ACQUITY UPLC H-Class PLUS Binary System using the adjusted method conditions are shown in Figure 2.
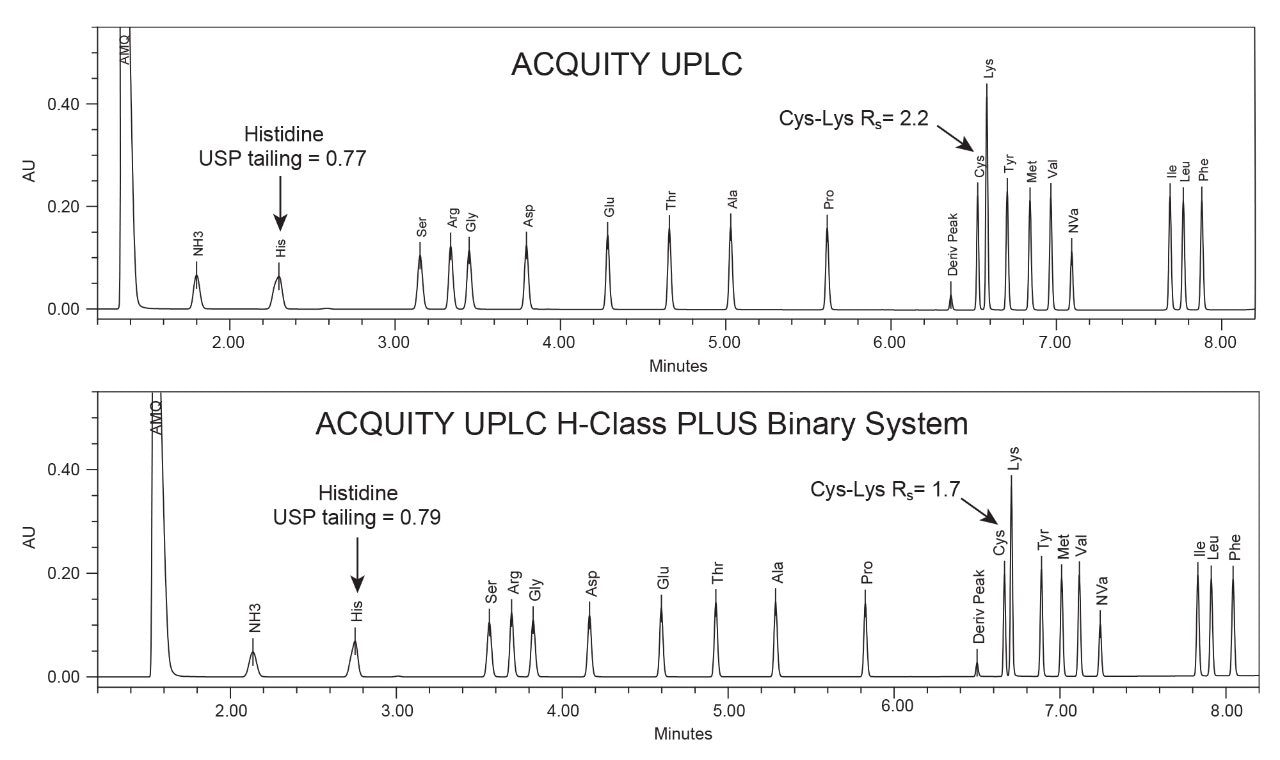
Two of the critical chromatographic requirements were adequate peak shape of the early eluting histidine peak and overall resolution of all peaks. Using the method modifications, the chromatograms obtained on the ACQUITY UPLC H-Class PLUS Binary System met both these requirements.
Verification of Hydrolysate Method Performance
Once the method conditions were adapted, the next step was to verify method performance on the ACQUITY UPLC H-Class PLUS Binary System to ensure equivalency with previously demonstrated results for the AccQ•Tag Ultra method on the ACQUITY UPLC.2 This included evaluation of linearity, limit of quantitation (LOQ), and intra- and interday precision. The data collected on the ACQUITY UPLC H-Class PLUS Binary System uses the method adjustments described above (30 µL needle, needle wash composition = 95:5 water:acetonitrile, and column temperature = 45 °C).
Linearity was evaluated over the previously established range of 1–500 µM for all amino acids (0.5–250 µM for cysteine). Norvaline was used as an internal standard and prepared at a mid-calibration range concentration of 250 µM. The calibration curve was generated from a single injection at each concentration level. Due to the large amount of data, R2 and calibration deviation results for four example compounds are shown in Table 1, and represent amino acids eluting at various sections of the chromatograms.
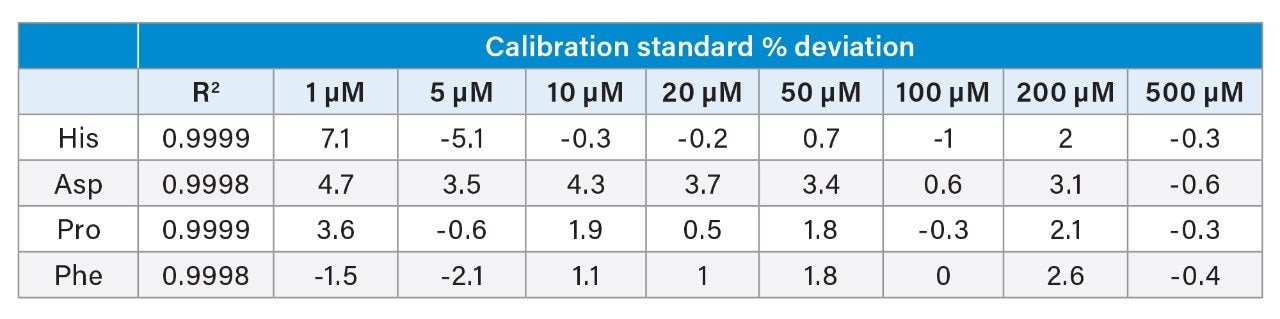
The adjusted method run on the ACQUITY UPLC H-Class PLUS Binary System generated very high coefficients of determination (R2), as well as very low % deviation from nominal concentration, especially at the limit of quantitation (1 µM, except cysteine at 0.5 µM) and low end of the calibration curve. This is evidence of a highly accurate and reliable quantitative method.
Both intra- and interday retention time and concentration precision were assessed by injecting 6 replicates of an amino acid standard sample prepared at the highest calibration concentration (500 µM, except cysteine at 250 µM) over three days. Table 2 shows the resulting intra- and interday precision values obtained on the ACQUITY UPLC H-Class PLUS Binary System.
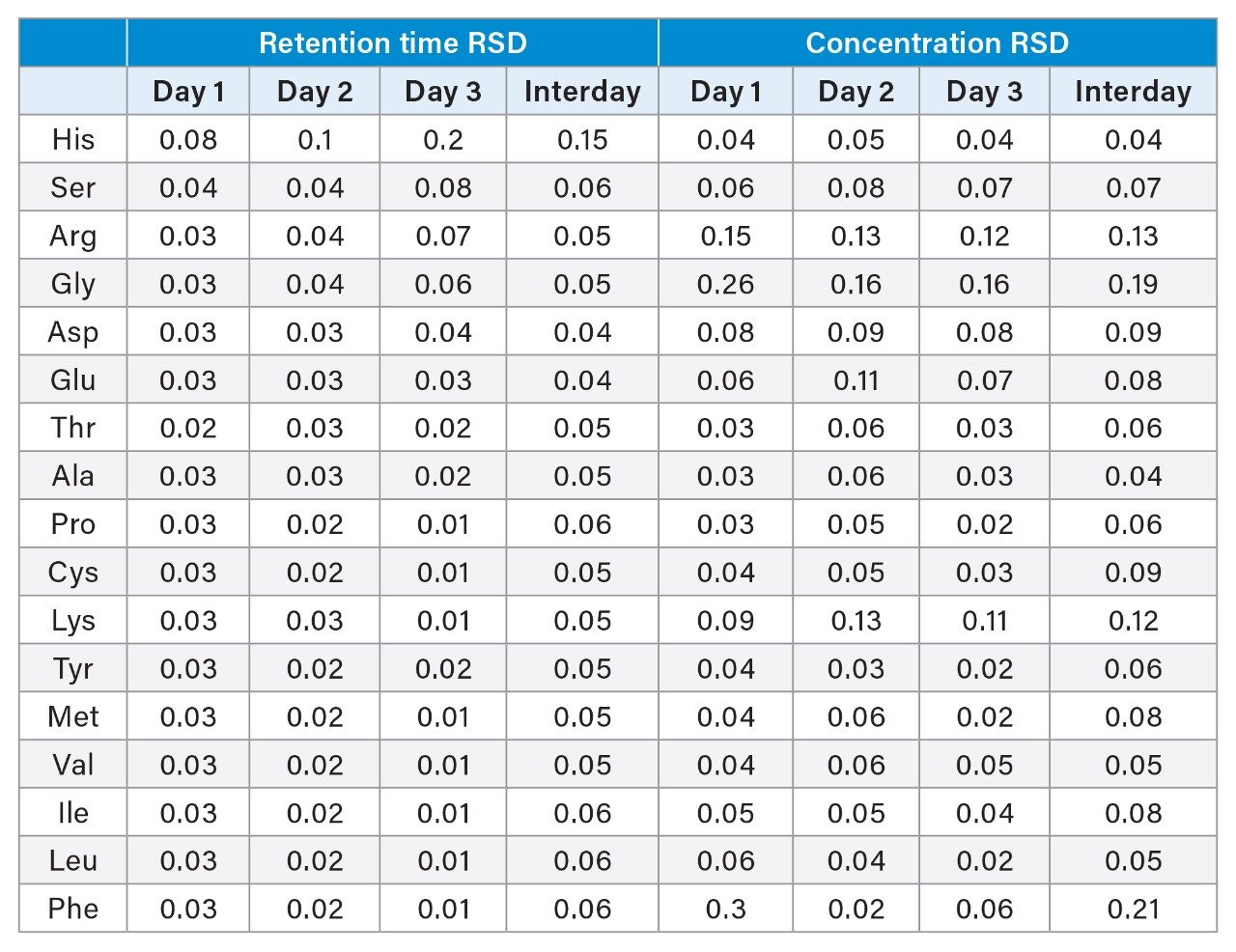
The retention time intra- and interday results show very low %RSD, indicating good repeatability. The highest retention time RSD seen over three days was only 0.2%, which corresponds to a retention time standard deviation of only 0.3 seconds. This highlights the very good gradient precision achievable on the ACQUITY UPLC H-Class PLUS Binary System. Additionally, the intra- and interday concentration results also show very low %RSDs for all amino acids, the highest overall being only 0.3% for 6 replicate injections. The interday precision, which is calculated for 18 total injections, 6 injections on each of three days, also shows very low %RSDs. The largest interday RSD obtained was only 0.2% measured across 3 days of analysis. The low intra- and interday precision results obtained on the ACQUITY UPLC H-Class PLUS Binary System is further evidence that the method adaptation has been successful.
Adaptation of Cell Culture, Food and Feed, and Alkylated Cysteine Methods
The method adjustments determined using the amino acid hydrolysate standard were then applied to methods used for analysis of cell culture media, foods and feeds, and alkylated cysteines. Amino acid analysis solutions have been developed for a variety of application areas, with each standard tailored to contain the relevant amino acids. In depth method verification, including evaluation of linearity, precision, and limit of quantitation, was not done for these methods. Rather, for each analysis method, only the chromatographic separation was verified using the appropriate standard and norvaline as the internal standard.
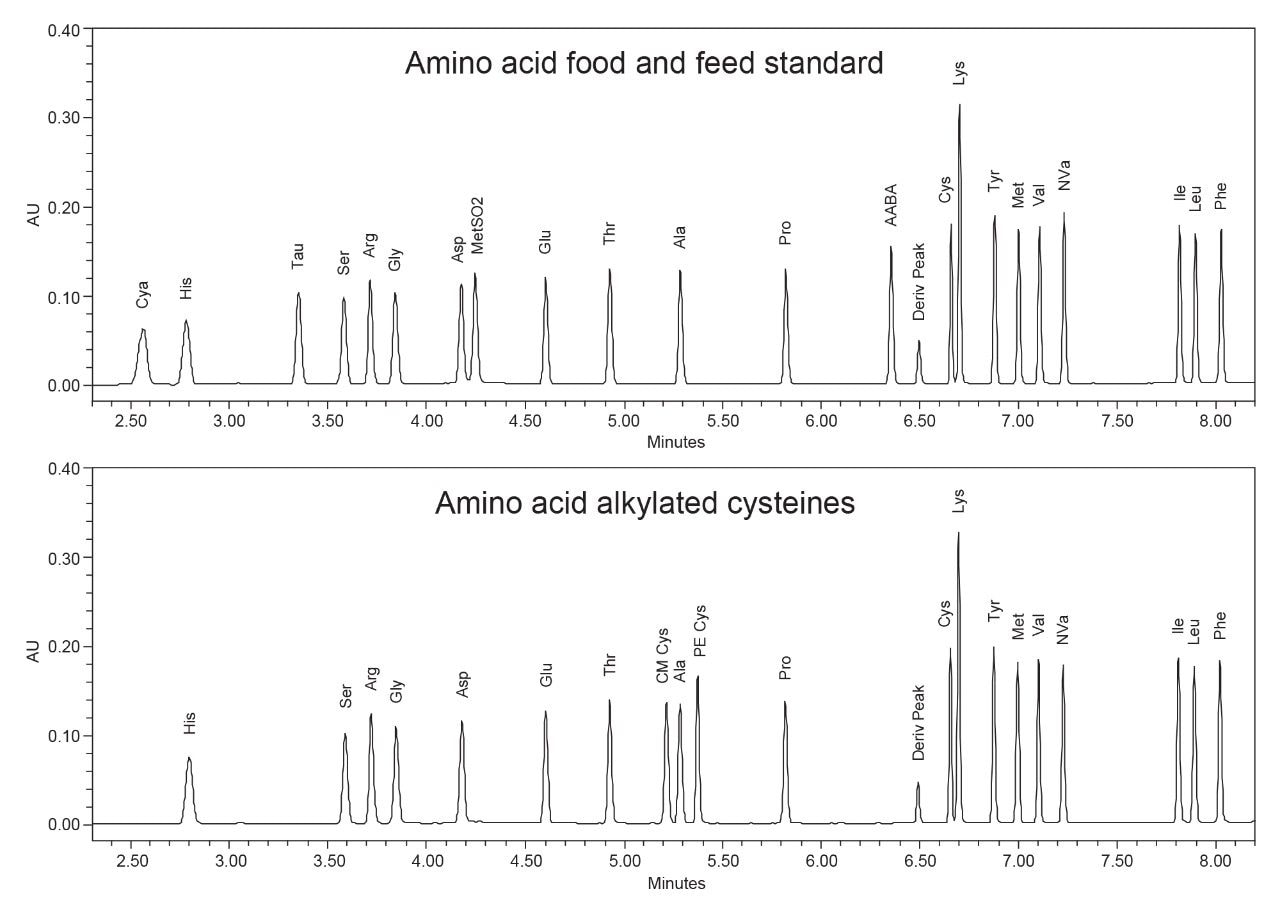
The commercially available amino acid food and feed standard and the manually prepared alkylated cysteine sample were analyzed using the same method and instrument conditions used for the amino acid hydrolysate standard. The amino acid food and feed standard contains the 17 amino acids of the hydrolysate plus taurine, α-aminobutyric acid (AABA), methionine sulfone (MetSO2), and cysteic acid. The addition of cysteic acid poses a potential challenge due to strong solvent effects given its early elution (before histidine), while the addition of MetSO2 can be challenging due to the required resolution with asparagine. With the modifications already in place to improve the histidine peak shape, the peak shape for cysteic acid was also very good with no further adjustments required. Additionally, the resolution of MetSO2 and asparagine was 1.7, which is adequate for most qualitative and quantitative methods and comparable to historical data acquired on the ACQUITY UPLC System.
For the alkylated cysteine sample, the 17 amino acid hydrolysate standard was used, plus the addition of pyridethylcysteine (PE Cys) and carboxymethylcysteine (CM Cys). Alkylation of cysteines is a common approach for analysis due to the instability of cysteine during hydrolysis, whereas the alkylated forms are stable and can be used for quantitation.3 The elution of these additional peaks is very close to alanine, so again we needed to ensure good resolution of all peaks when analyzed with the method adjustments. The resulting resolution values for CM Cys-Ala and Ala-PE Cys were 1.9 and 2.6 respectively, which are both acceptable for qualitative and qualitative work (Figure 3).
The final amino acid application that was examined on the ACQUITY UPLC H-Class PLUS Binary System was the amino acid cell culture standard. This sample uses a more concentrated Eluent A (see UPLC Amino Acid Analysis Guide) for separation and has the 17 amino acids of the hydrolysate standard plus an additional 9 amino acids. The column temperature was set to 50 °C, and the system set up also included the 30 µL injection needle, and a wash solvent of 95:5 water:acetonitrile. A representative chromatogram of the amino acid cell culture standard using norvaline as the internal standard is shown in Figure 4.
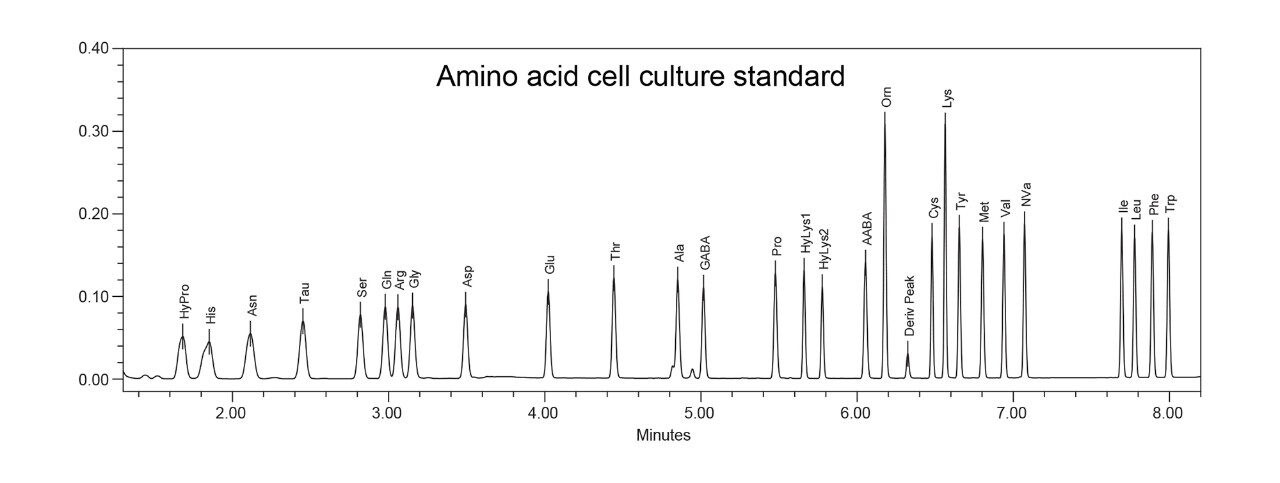
With the additional 9 amino acids in the cell culture standard, the resolution of several critical pairs was an important requirement using the adjusted method parameters. The addition of glutamine was challenging due to the elution within the triplet of serine/arginine/glycine. The adjusted method conditions yielded resolutions of 2.7, 1.4, and 1.7 for Ser-Gln, Gln-Arg, and Arg-Gly respectively, which is nearly identical to the resolutions obtained using the ACQUITY UPLC and the original method.4 The other additional amino acids did not present any difficulty in obtaining adequate resolution. The addition of hydroxyproline presented another early eluting compound that needed adequate separation with histidine and symmetrical peak shape with no impact of strong solvent effects. Using the modified method conditions, the resolution between histidine and hydroxy proline was 1.4 and both yielded symmetric peak shapes. The overall results are again commensurate with the original method developed on the ACQUITY UPLC System.
Sample Analysis of an Energy Drink
To reinforce the successful method adaptation from the original methods developed on the ACQUITY UPLC to the method conditions run on the ACQUITY UPLC H-Class PLUS Binary System, a quantitative example was compared across the two systems. A quantitative method to analyze the amount of taurine in two energy drink samples was employed. A calibration curve spanning the range of 10–500 µM was prepared using the food and feed standard and norvaline as the internal standard. The unknown samples were diluted 1:10, 1:100, and 1:200 prior to derivatization. The taurine response for the 1:10 diluted sample was above the highest calibration standard, therefore the results for the remaining two samples are presented in Table 3.
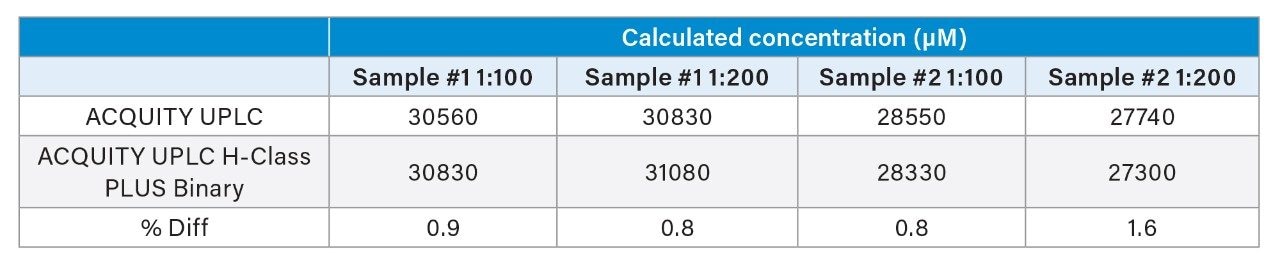
The quantitative results obtained on the two LC systems show very good agreement between values, with the highest difference of the four sample preparations being only 1.6%. This further confirms that the method adaptation on the ACQUITY UPLC H-Class PLUS Binary System has been successful is suitable for quantitative analysis.
Conclusion
In the pharmaceutical and biopharmaceutical industry, it is very common for methods to be used for many years, often decades. Additionally, there is a natural evolution of development of new instrumentation and obsolescence of older technologies. It is critical that methods can be adapted to be run on new instrumentation without loss of critical qualitative and/or quantitative performance. In this application note, the original ACQUITY UPLC methods developed for amino acid analysis have been successfully adapted for the ACQUITY UPLC H-Class PLUS Binary System with no loss of performance. Critical parameters such as peak shape, resolution, linearity, precision, and limit of detection were conserved between the systems. Finally, quantitative analysis of taurine in energy drinks yielded nearly identical results for the ACQUITY UPLC and the ACQUITY UPLC H-Class PLUS Binary System, demonstrating the successful method adaptation.
References
- Amino Acid Standard Kits. Waters Care and Use Manual. 720006663EN, 2020.
- Amino Acid Analysis Application Notebook. 720006130EN, 2018.
- Cohen, S. Analysis of Sulfur Containing Amino Acids III. Alkylation of Cysteine. Waters Lab Highlights. LAH0379, 1988.
- Hong, P, Wheat, TE, Mazzeo, JR, Diehl, DM. Monitoring Cell Culture Media with the Waters Amino Acid Analysis Solution. Waters Application Note 720002381EN, 2007.
720007368, September 2021