Reductions in Cost and Time by Modernizing a USP Monograph Method from HPLC to UHPLC and UPLC Instrumentation and Columns
Abstract
Modernization of the USP assay method for naproxen sodium tablets was performed to demonstrate the potential cost and time savings. Modernization of the method can drastically reduce both analysis time and mobile phase usage. To highlight this, a lifetime study of the UPLC column was performed and the results showed no change in performance over 10,000 injections of a naproxen sodium sample. Using the analysis run times and flow rates, estimated costs of the mobile phase needed for 10,000 injections were calculated for the HPLC, UHPLC, and UPLC methods. Up to an 8x decrease in analysis time and a 13x decrease in mobile phase cost may be realized by modernizing the HPLC assay of naproxen sodium tablets to UHPLC or UPLC instrumentation and columns.
Benefits
- Four-fold reduction of analysis time and solvent usage by modernizing the HPLC method to UHPLC instrumentation and columns
- Up to a thirteen-fold decrease in solvent usage and an eight-fold decrease in analysis time by modernizing the HPLC method to UPLC instrumentation and columns
- A potential cost savings of $10,000 and a 48-day decrease in testing time over the life of the column (10,000 injections) were calculated for the UPLC versus the HPLC method
Introduction
The United States Pharmacopeia, USP, is a compendium of methods that are used for testing a variety of pharmaceutical products. USP monographs typically describe methods for impurity analyses, dissolution testing, and other assays,1-3 using techniques ranging from thin layer chromatography (TLC), to gas chromatography, and liquid chromatography. However, many of these methods are now considered “outdated” as they employ older equipment that lacks the resolving power and speed of modern technologies. Significant improvements can be achieved by modernizing these methods to newer technology. This modernization process is governed by USP <621>, a general chapter that guides permissible changes in liquid chromatography testing conditions including variations in column configuration, particle size, flow rate, and injection volume.4 By following <621> guidelines, a method can be modernized without the need for re-validation.5
In this application note, we demonstrate modernization of a USP method for naproxen sodium tablets from HPLC to UHPLC and UPLC technologies. Naproxen sodium is a common, non-steroidal, anti-inflammatory drug (NSAID) sold over the counter in both brand name, Aleve, as well as generic formulations. Quality control testing for batches of naproxen sodium must take place regularly to ensure properly delivery of the compound to help reduce pain and inflammation. Here, a 4.6 x 150 mm 5 μm column was replaced by narrower and shorter columns containing smaller particles. To further demonstrate the benefits of this modernization, a lifetime study was performed for a UPLC column. The results were used to calculate the cost savings over the lifetime of the column due to the reduced mobile phase consumption for the UPLC method vs its HPLC counterpart.
Experimental
Sample Description
Five tablets (220 mg each) of naproxen sodium were crushed and dissolved in 1 liter of mobile phase:water (85:15). The solution was filtered with a 0.2 µm nylon filter and diluted 10x with mobile phase for a final sample concentration of 0.11 mg/mL as per the USP monograph sample preparation.
Standard Description
Naproxen sodium stock solution was created using neat naproxen sodium powder and mobile phase as the sample diluent. The stock solution was diluted 10x with mobile phase to a final standard concentration of 0.11 mg/mL.
Method Conditions
|
LC Conditions |
|
|
LC systems: |
Alliance HPLC with 2489 UV/Vis Detector ACQUITY Arc with 2998 PDA Detector ACQUITY UPLC H-Class with PDA Detector |
|
Detection: |
UV @ 254 nm |
|
Vials: |
LCMS Certified Clear Glass Vial 2 mL (PN: 600000751CV) |
|
Column(s): |
XBridge BEH C8, 5 µm, 4.6 x 150 mm (PN: 186003017) XBridge BEH C8 XP, 2.5 µm, 3.0 x 75 mm (PN: 186006046) CORTECS UPLC C8, 1.6 µm, 2.1 x 50 mm (PN: 186008399) |
|
Column temp.: |
30 °C |
|
Sample temp.: |
10 °C |
|
Flow rate: |
1.20 mL/min (Alliance) 1.02 mL/min (ACQUITY Arc) 0.74 mL/min (ACQUITY UPLC H-Class) |
|
Mobile phase composition: |
Acetonitrile:water:acetic acid (45:54:1 v/v/v) |
|
Injection volume: |
20 µL (Alliance) 4.3 µL (ACQUITY Arc) Values for the ACQUITY Arc and ACQUITY UPLC H-Class were calculated using the ACQUITY Column Calculator 1.4 µL (ACQUITY UPLC H-Class) |
|
Data Management |
|
|
Chromatography software: |
Empower 3 Feature Release 5 |
Results and Discussion
To validate the USP monograph method prior to modernization, an Alliance HPLC System with a 2489 UV/Vis detector was equipped with an appropriate L7 column – an XBridge BEH C8, 5 µm, 4.6 x 150 mm Column. The average peak areas (n=10), or responses were calculated separately for the samples and standards and used in the calculation of naproxen sodium content. The equation for calculating this is shown in Figure 1, where ru is the response, or peak area, of the naproxen sodium sample, rs is the response of the standard, Cs is the concentration of the standard, and Cu is the concentration of the sample. For the assay to pass the assay result must be between 90–110%.
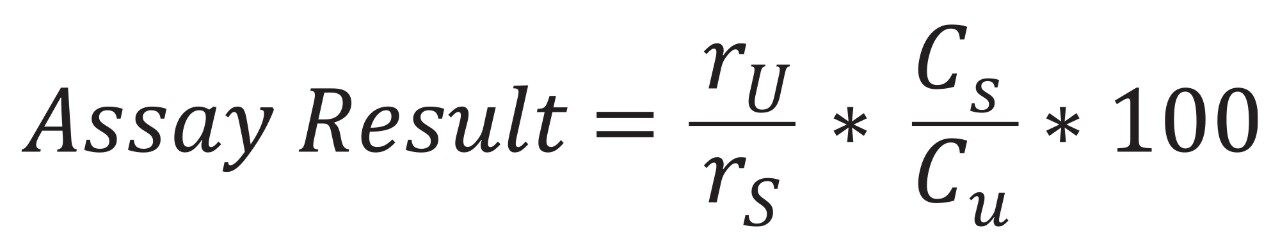
Since both the standard and sample were made to a concentration of 0.11 mg/mL the Cs and Cu values cancel each other out, leaving only the ratio of responses. Along with the assay result, relative standard deviations for naproxen peak area must be no more than 2% and the USP tailing factor for naproxen can be no more than 2.0 for both the original test conditions and modernized methods. Figure 1 shows representative chromatograms of naproxen obtained using the columns and systems tested.
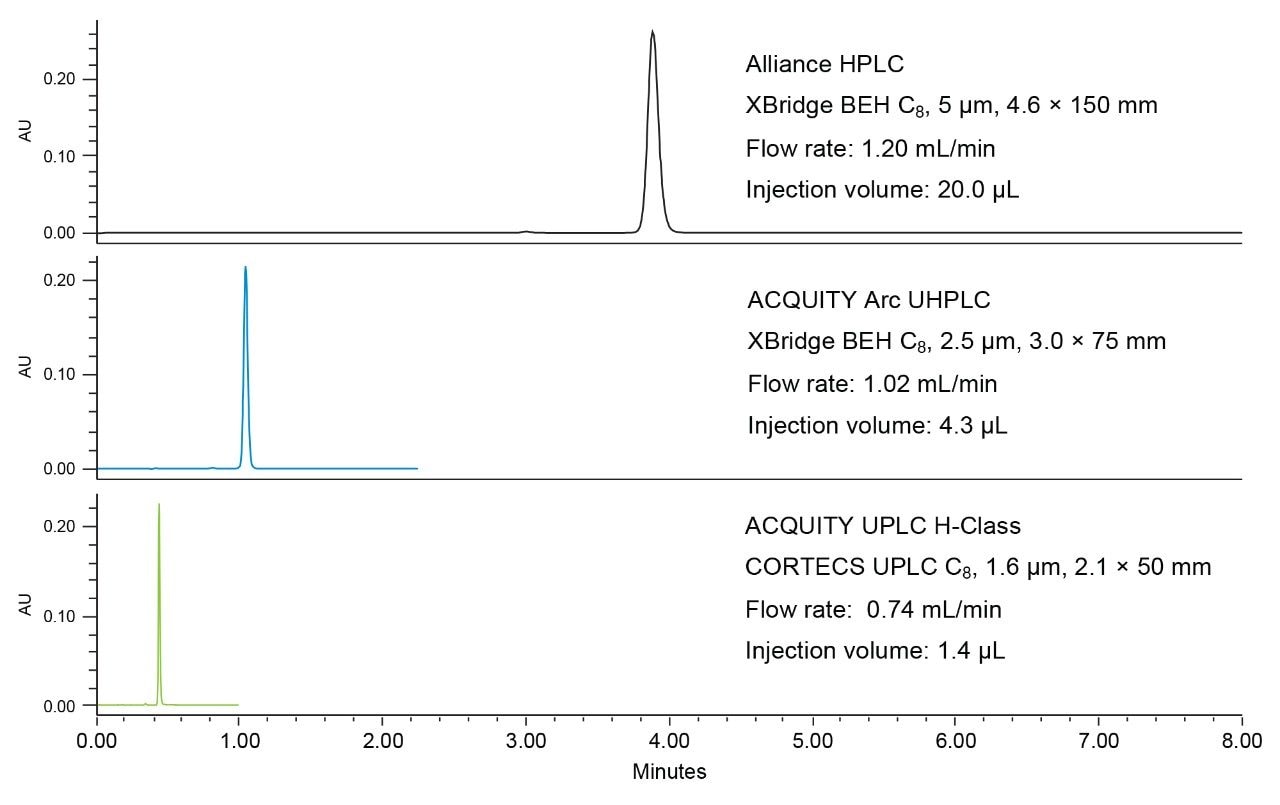
All systems and columns tested passed the assay criteria as outlined in Table 1. To modernize the HPLC conditions to the UHPLC system, a 3.0 x 75 mm column packed with 2.5 µm particles was used to maintain a constant L/dp ratio. The column selected was an XBridge BEH C8 Column. This column uses the same particle and bonding technology as the original HPLC column tested, with a smaller particle size. To modernize to UPLC, sub 2 µm particles in 2.1 x 50 mm hardware were used. In this example, a CORTECS UPLC C8 1.6 µm, 2.1 x 50 mm Column was installed on an ACQUITY UPLC H-Class System. While an ACQUITY UPLC BEH C8 Column would also be appropriate, CORTECS Columns are specifically designed to improve column efficiency.7–10
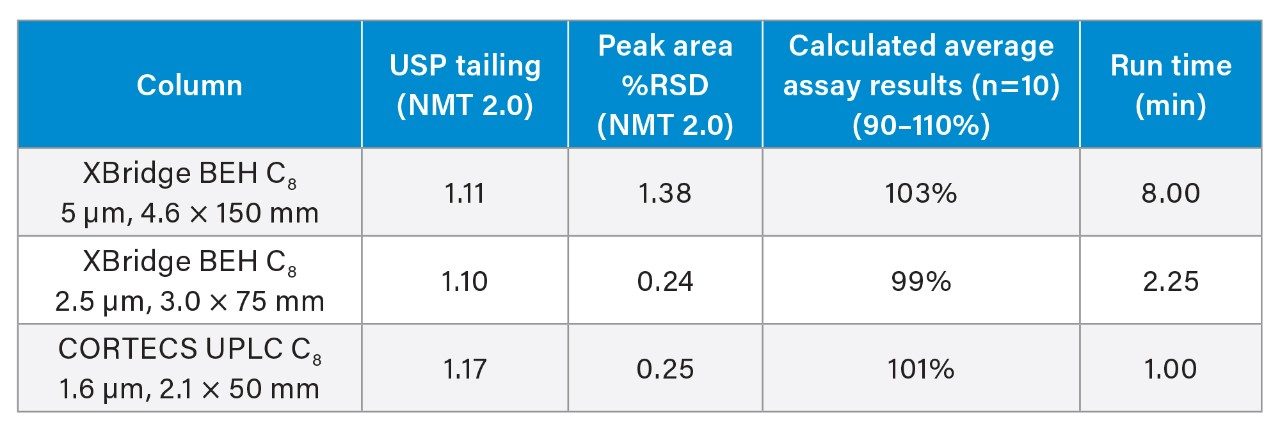
We also assessed the time and cost savings of an assay in relation to the lifetime of a column, or the number of injections expected before the assay fails and the column needs to be replaced. An ACQUITY UPLC H-Class System equipped with a CORTECS UPLC C8 Column was selected for the lifetime study in an effort to use the lowest amount of solvent and achieve the fastest run time. The system was set up to run non-stop, performing replicate injections of the standard (n=5) followed by replicate injections of the sample (n=100). This sequence was chosen because the naproxen sodium sample is more likely to cause column failure due to the presence of excipients. The injections of the naproxen standard were to ensure system suitability requirements were still met. USP tailing factor and system pressure were closely monitored as these two parameters are among the first to fail during a lifetime test. The lifetime study was halted after 10,000 injections were performed without loss of column performance or increase in column pressure. Figures 2 and 3 show the system pressure and USP tailing factor for naproxen taken from every 100 injections of the naproxen sodium sample.
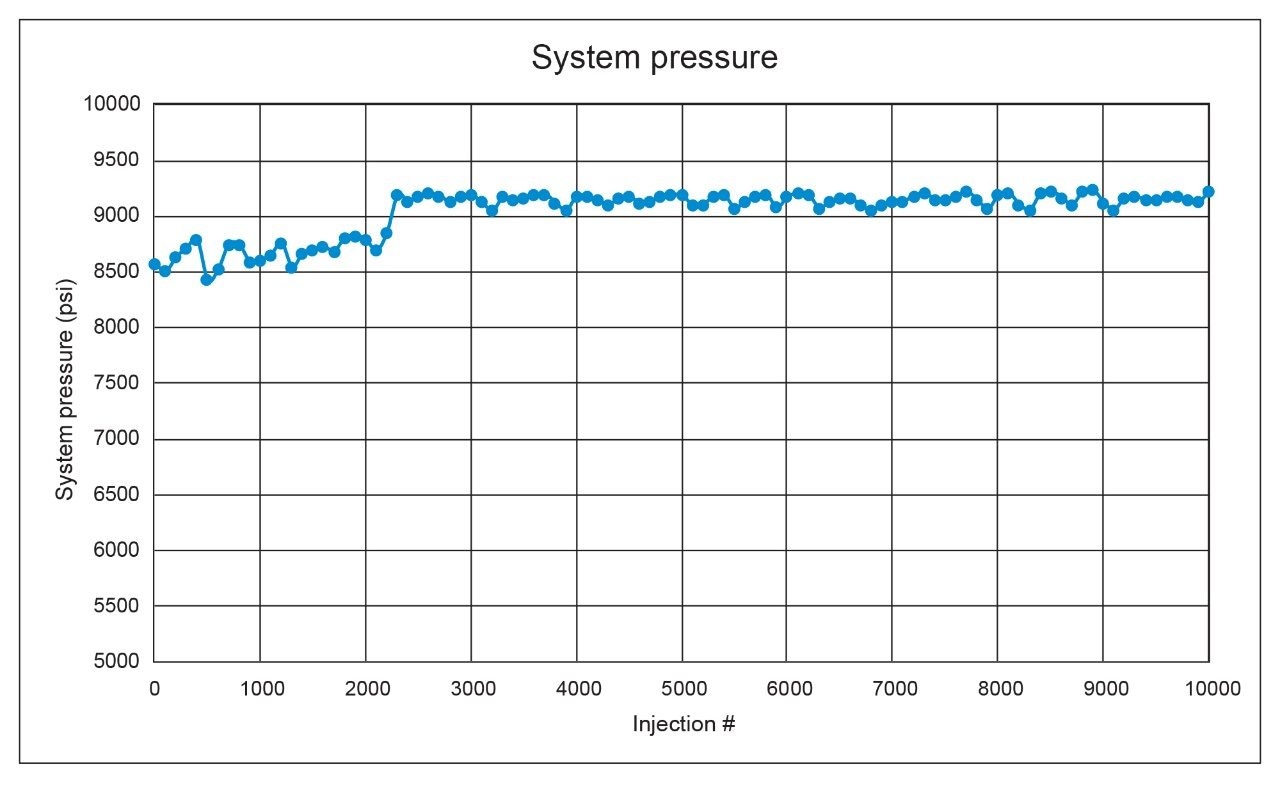
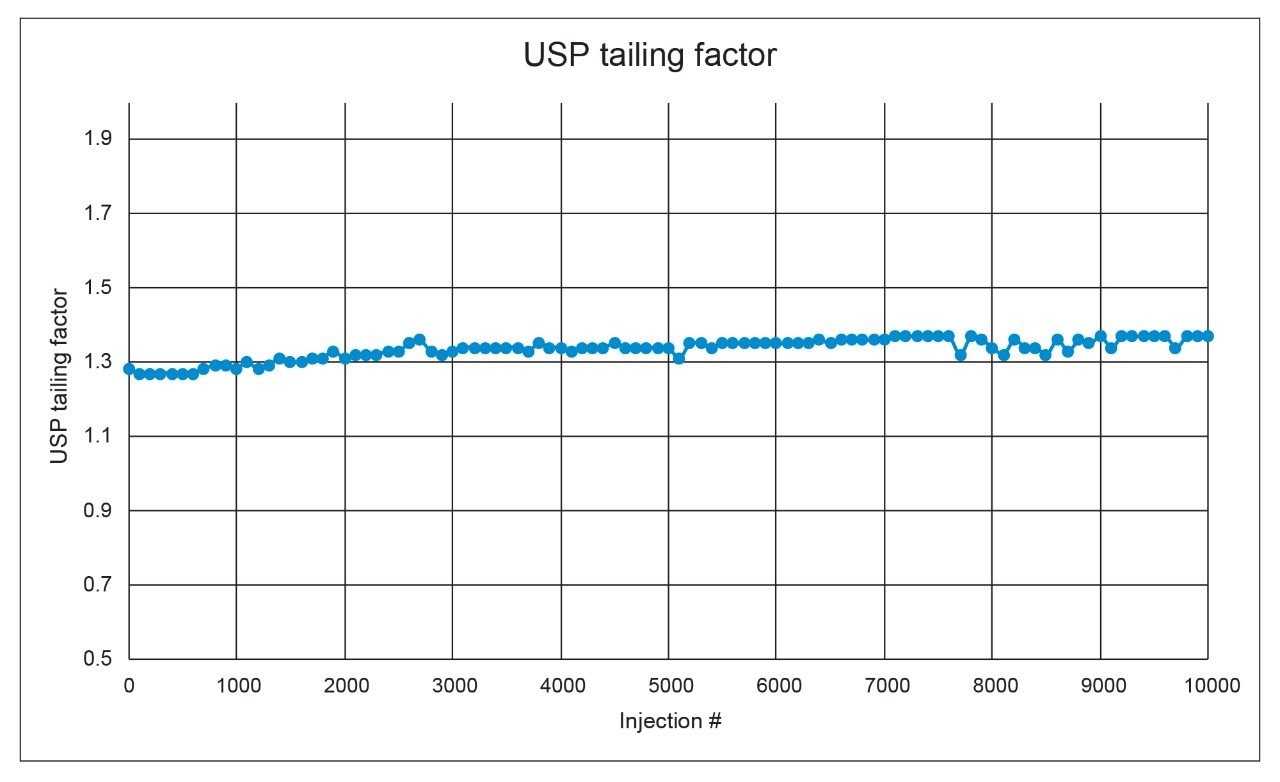
Though a system leak in the solvent manager caused the column to see lower pressures initially until fixed, the assay results, relative standard deviations, and USP tailing factors were within specifications throughout the testing. A slight increase in USP tailing was observed over time, however the results were still well below the 2.0 limit for USP tailing. The system pressure was also stable across the 10,000 injections with only slight variations in pressure detectable, which is different than the expected increase due to column failure.11 Typically during a lifetime study, the excipients in the formulated drug product will foul the inlet frit of the column leading to steadily increasing system pressure and decreased column performance in the form of peak shape degradation. Given that the column lasted 10,000 injections, a rough cost and time estimate can be determined. Table 2 shows rough estimates for solvent cost over the lifetime of the column using the three different system types and testing conditions.
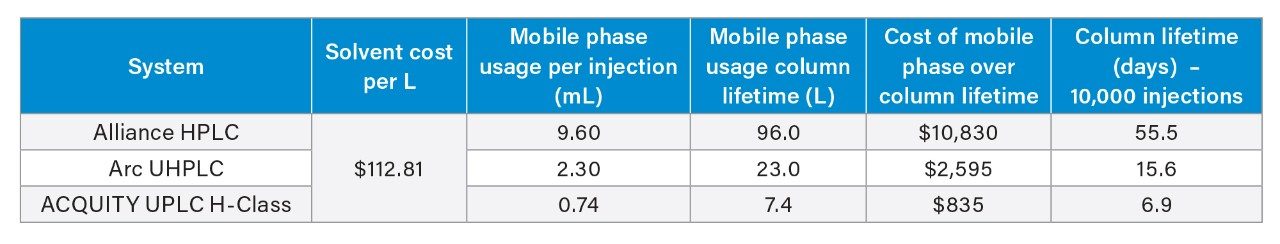
The mobile phase cost was based on prices of acetonitrile, water, and glacial acetic acid found on the Sigma Aldrich website and the composition of the mobile phase.12-14 Cost of mobile phase per L was calculated as indicated.15
These costs do not factor in disposal of waste, storage of solvents, or analyst time. As shown in Table 2, the use of the older technology HPLC system and column is almost 5x more expensive than using UHPLC instrumentation. Moreover, UPLC technology grants potential savings of up to $10,000 for the naproxen sodium assay when compared to using HPLC over 10,000 injections. This is combined with a time savings of 48 days when comparing the HPLC assay conditions to the UPLC counterpart.
Conclusion
Methods detailed in USP monographs often employ older and outdated technologies. The use of larger columns, large particle size stationary phases, and HPLC instrumentation can be modernized to newer instrumentation and particle technologies through guidelines as provided by general chapter <621>. We demonstrated the value of modernization using the USP assay method for naproxen sodium tablets. We achieved drastically reduced analysis times and mobile phase costs by scaling the original HPLC assay conditions to an ACQUITY Arc UHPLC System and an ACQUITY UPLC H-Class System using smaller particle size stationary phases and shorter and narrower columns. Over the lifetime of the column (estimated as 10,000 injections), the reduction in mobile phase usage may save up to $10,000 while the reduced run time could cut the time of analysis by almost 48 days.
References
- Baksam V, Nimmakayala S, Devineni SR, Muchumarri RM, Shandilya S, Kumar P. Isolation and Characterization of Thermal Degradation Impurity in Brimonidine Tartrate by HPLC, LC-MS/MS, and 2DNMR. Journal of Pharmaceutical and Biomedical Analysis. 2021. (205) 114297.
- Aliyu AO, Garba S, Balogun LO, Awe FE. Quality Assessment of Some Selected Brands of Amoxicillin Clavulanate from Pharmaceutical Stores in Kaduna Metropolis, Nigeria. Journal of Pharmacy and Biological Sciences. 2021-Apr. (16) 33–40.
- Monisha ST, Ela KN, Islam R, Ether SA, Rahman FI. Quality Attributes Comparison of Selected Brands of Rosuvastatin Calcium Tablets Marketed in the US and Bangladesh. Journal of Pharmaceutical Research International. 2021 (33) 46–55.
- 621-Chromatography.pdf Accessed 9-Sept-2021. https://www.bioglobax.com/wp-content/uploads/2018/08/621-Chromatography.pdf.
- Swann T, Nguyen JM. USP Method Modernization Using “Equivalent L/dp” and “Equivalent N” Allowed Changes with CORTECS C8 and CORTECS UPLC C8 Columns. Waters Application Note. 2016, 720005666EN.
- Naproxen-sodium-tablets.pdf. Naproxen Sodium Tablets USP monograph. Accessed 9-Sept-2021. https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/iras/naproxen-sodium-tablets.pdf.
- Berthelette KD, Summers M, Fountain KJ. Improving Resolution using CORTECS UPLC Columns. Waters Application Note. 2013, 720004737EN.
- Berthelette KD, Nguyen JM, Turner JE. Improving Peak Capacity while Maintaining Selectivity using CORTECS Columns on an Agilent 1290 LC system. Waters Application Note. 2021, 720007250EN.
- Shia JC, Yabu M, Tran KV, Young M. Improved Resolution of Paraquat and Diquat: Drinking Water Analysis Using the CORTECS UHPLC HILIC Column. Waters Application Note. 2013, 720004732EN.
- Danaceau JP, Chambers EC, Fountain KJ. Advantages of CORTECS C18 2.7 µm and XBridge BEH Phenyl XP 2.5 µm Columns for the Analysis of a Comprehensive Panel of Pain Management Drugs for Forensic Toxicology. Waters Application Note. 2014, 720005185EN.
- Berthelette KD, Fountain KJ, Summers M. Improving Method Robustness for Routine Analysis of Pharmaceutical Formulations. Waters Application Note. 2013, 720004807EN.
- Acetonitrile Suitable for HPLC, gradient grade ≥99.9% Product page. Cost of 1L: $185.00. https://www.sigmaaldrich.com/US/en/product/sigald/34851?context=product.
- Water Suitable for HPLC Product page. Cost of 1 L: $51.20. https://www.sigmaaldrich.com/US/en/product/sigald/270733?context=product.
- Acetic Acid, Glacial HPLC, Meets ACS Specifications Product Page. Cost of 500 mL: $87.90. https://www.sigmaaldrich.com/US/en/product/mm/ax0074?context=product.
- Calculation of cost per L of mobile phase.
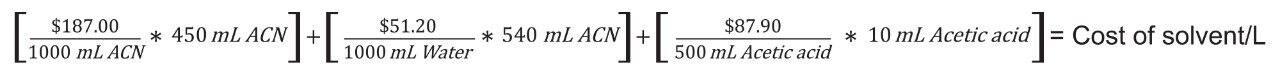
720007427, November 2021