Analysis of Aflatoxins in Corn and Peanuts Using Immunoaffinity Chromatography and the Arc™ HPLC System
Abstract
Aflatoxins are carcinogenic compounds produced by fungi, and are controlled worldwide via regulation, including maximum levels in food and feed. This application note describes the improvement of an existing AOAC method using the Waters Arc HPLC System with fluorescence detection. Effective sample clean-up step was achieved using AflaTest™ WB immunoaffinity chromatography (IAC). Recoveries in corn or peanuts were greater than 90%, thus meeting the EU criteria. The method has been demonstrated to be suitable for checking compliance with regulatory limits.
Benefits
- Waters new Arc HPLC System can be used to successfully analyze aflatoxins, meeting or exceeding the conditions from those used previously with AOAC methods
- The Arc HPLC System can run at faster flow rates giving shorter run times and the option of not using a photochemical reactor
- The original protocol used for AflaTest immunoaffinity column clean-up has been improved with decreased sample preparation time, and aflatoxin recoveries meeting regulatory acceptance criteria
- The Arc HPLC conditions in this work can be used to analyze aflatoxin in other types of foods or feed, including baby foods
Introduction
Aflatoxins are a group of fungal metabolites produced by several strains of Aspergillus, which occur in many foods, such as cereals, spices, nuts, and dried fruit, etc.1–3 Fungi can contaminate raw agricultural commodities during pre- and post-harvest conditions and can also be present in finished food products. Aflatoxins are responsible for both toxicity and carcinogenicity in humans and other mammals, which are called aflatoxicoses. The main aflatoxins of interest are aflatoxins B1, B2, G1, and G2.1 Aflatoxin B1 is the most abundant and toxic compound in this group, ranked by the World Health Organization International Agency for Research on Cancer as a group 1 carcinogen.
Due to concerns over impacts on both human and animal health caused by aflatoxin contamination in food and feed, many countries have issued regulations to control the level of aflatoxins including maximum permitted levels of aflatoxin.4 A recent review of contemporary published papers in the field showed a high number of aflatoxin contaminations in food at levels that exceed a regulatory limit of 20 µg/kg and 4 µg/kg set for foods for human consumption in the USA and European Union, respectively.5 This emphasizes the need for increased analytical testing, as an effective strategy for prevention, control, and periodic monitoring of mycotoxin in all stages from field to the consumer.
High Performance Liquid Chromatography (HPLC) with fluorescence detection (FLD) is an important analytical technique used to determine the concentration of aflatoxins in food and feed, after liquid-liquid extraction and IAC cleanup. Analysis has been demonstrated previously using both HPLC (Alliance) and UPLC (ACQUITY) platforms, using fluorescence detection, supported with post-column derivatization for HPLC.6 The Arc HPLC is a quaternary-based LC system that can be used to improve the performance of an existing method through the benefits of minimal carryover, a higher backpressure limit, and automated priming and system preparation.7,8 The high-pressure limit of the Arc HPLC system allows the use of high flow rates with smaller particle columns to gain efficiency and reduce the analytical run time.
The objective of this work was to transfer an existing AOAC method for aflatoxin analysis to the new Arc HPLC System, and to establish the performance of the improved method at the very low concentrations needed for checking compliance with regulatory limits for aflatoxins in food or feed.
Experimental
Sample Description and Preparation
Corn and peanuts were obtained commercially and ground prior to extraction.
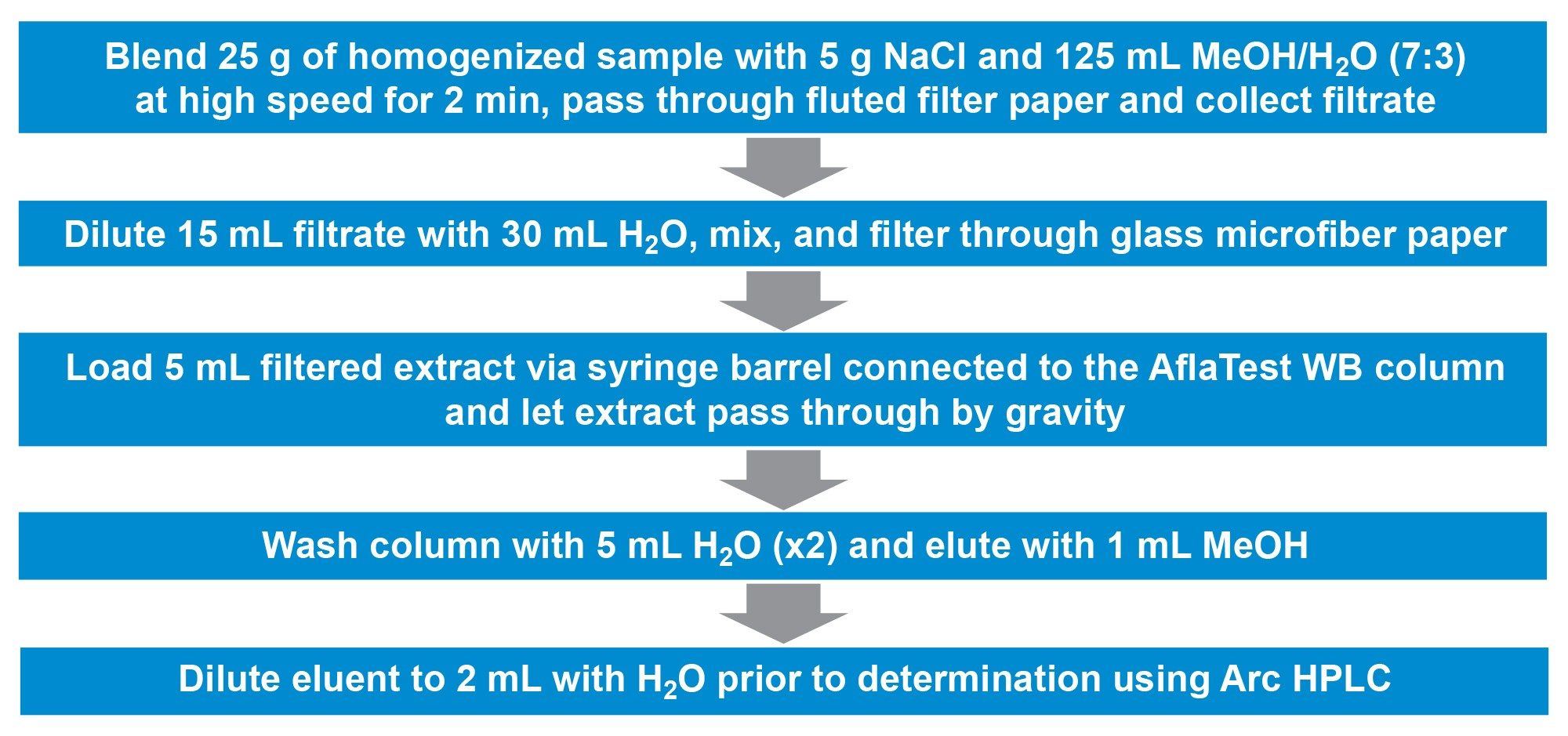
A modified version of the official AOAC method 991.31 was used in this study.9 The AflaTest WB (VICAM Cat. No. G1024), an IAC cleanup column designed to speed up aflatoxins analysis, meets the aflatoxin immunoaffinity column requirements in AOAC official method 991.31. An overview of the details of sample extraction and clean-up for aflatoxins is given in Figure 1. Ground corn or peanut homogenate were blended with sodium chloride (NaCl) and a mixture of methanol (MeOH) and water (H2O). After dilution, the extract was applied to the AflaTest WB IAC column, containing specific antibodies to aflatoxin, washed and aflatoxins eluted. Subsequent determination was done by using the Arc HPLC with FLD. More details on the AflaTest WB column can be found at https://www.vicam.com/products/aflatest-wb.
Aflatoxin standard containing aflatoxin B1, B2, G1 and G2 at 1, 0.3, 1 and 0.3 µg/mL, respectively, was purchased from Sigma-Aldrich (St. Louis, MO). Aflatoxins standards at various concentrations were prepared in methanol:water (50:50, v/v) daily.
LC Conditions
|
LC system: |
Arc HPLC |
|
Detection: |
PhCR Photochemical Reactor (VICAM p/n: 600001222) and 2475 Fluorescence (FLR) Detector with excitation 360 nm and emission 440 nm |
|
Vials: |
Deactivated Amber Glass 12 x 32 mm Screw Neck Vial, 2 mL (p/n: 186000846DV) |
|
Column(s): |
XSelect HSS T3 Column, 100 Å, 3.5 µm, 4.6 x 150 mm (p/n: 186004786) |
|
Column temp.: |
40 °C |
|
Sample temp.: |
15 °C |
|
Injection volume: |
50 µL |
|
Flow rate: |
1 mL/min |
|
Mobile phase: |
Isocratic (55:45 aqueous (water):organic (methanol), v/v) |
|
Run time: |
12 min |
Data Management
|
Chromatography software: |
Empower 3 (CDS) |
The signal-to-noise ratio (S/N) was calculated using the Empower Personal System Suitability. Limit of detection and quantification were estimated for a S/N of 3 and 10, respectively. Corn meal or peanut homogenate were spiked with 0.5, 4, and 50 µg/kg total aflatoxin (ratio of aflatoxins B1:B2:G1:G2 1:0.3:1:0.3), with each concentration in triplicate. Aflatoxins in corn meal or peanut homogenate without spiked aflatoxins were analyzed using the same procedure as blanks. The recovery of aflatoxin was calculated as: (Calculated concentration in spiked sample - Calculated concentration in blank sample) / spiked concentration) *100. Concentration of aflatoxin was calculated using a standard curve in solvent. Analysis of variance (ANOVA) was used to examine the difference in the recovery of each aflatoxin. The significance level of α= 0.05 was used and these statistical tests were performed using SAS (Version 9.4, SAS Institute Inc. Cary, NC).
Results and Discussion
Method Transfer to Arc HPLC
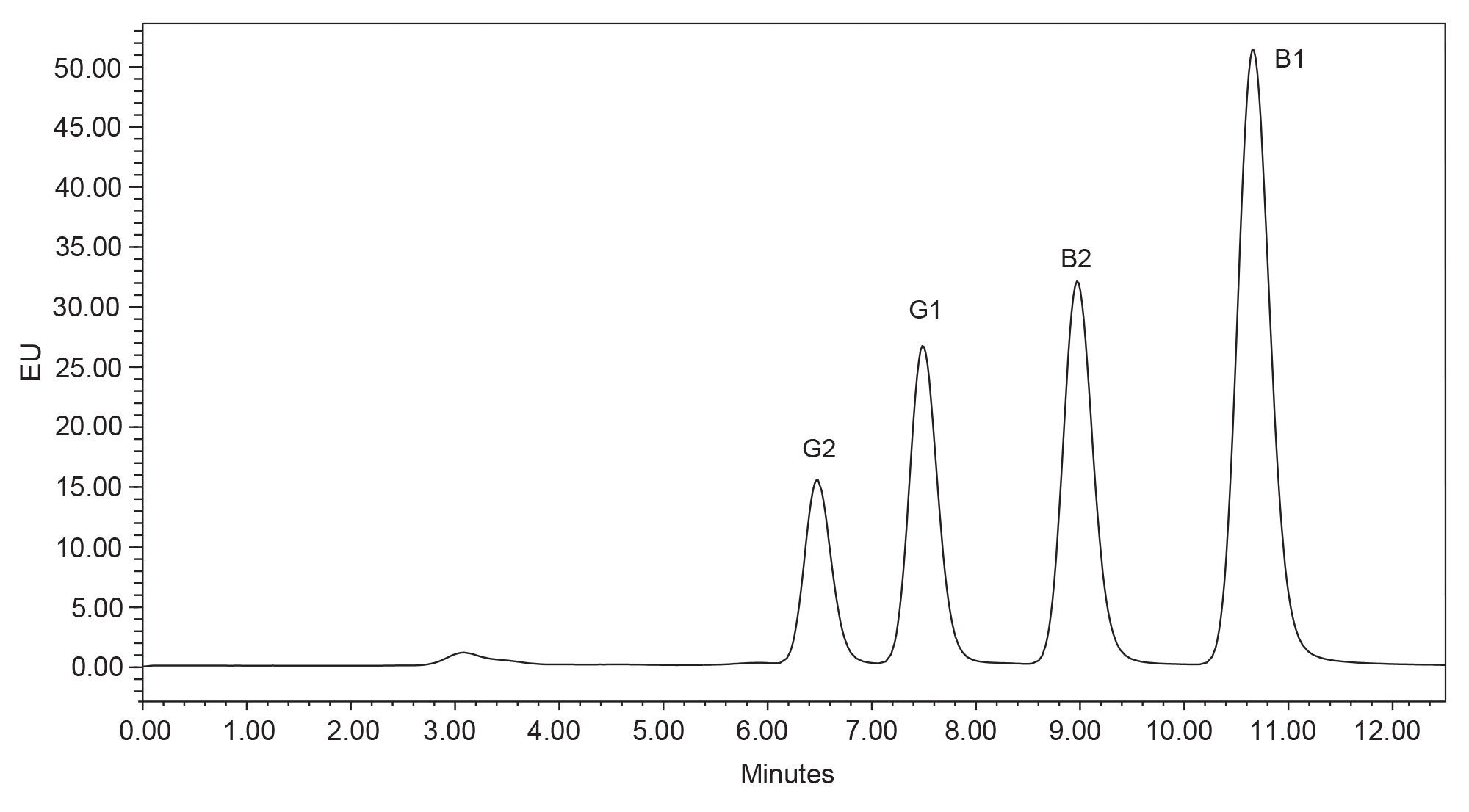
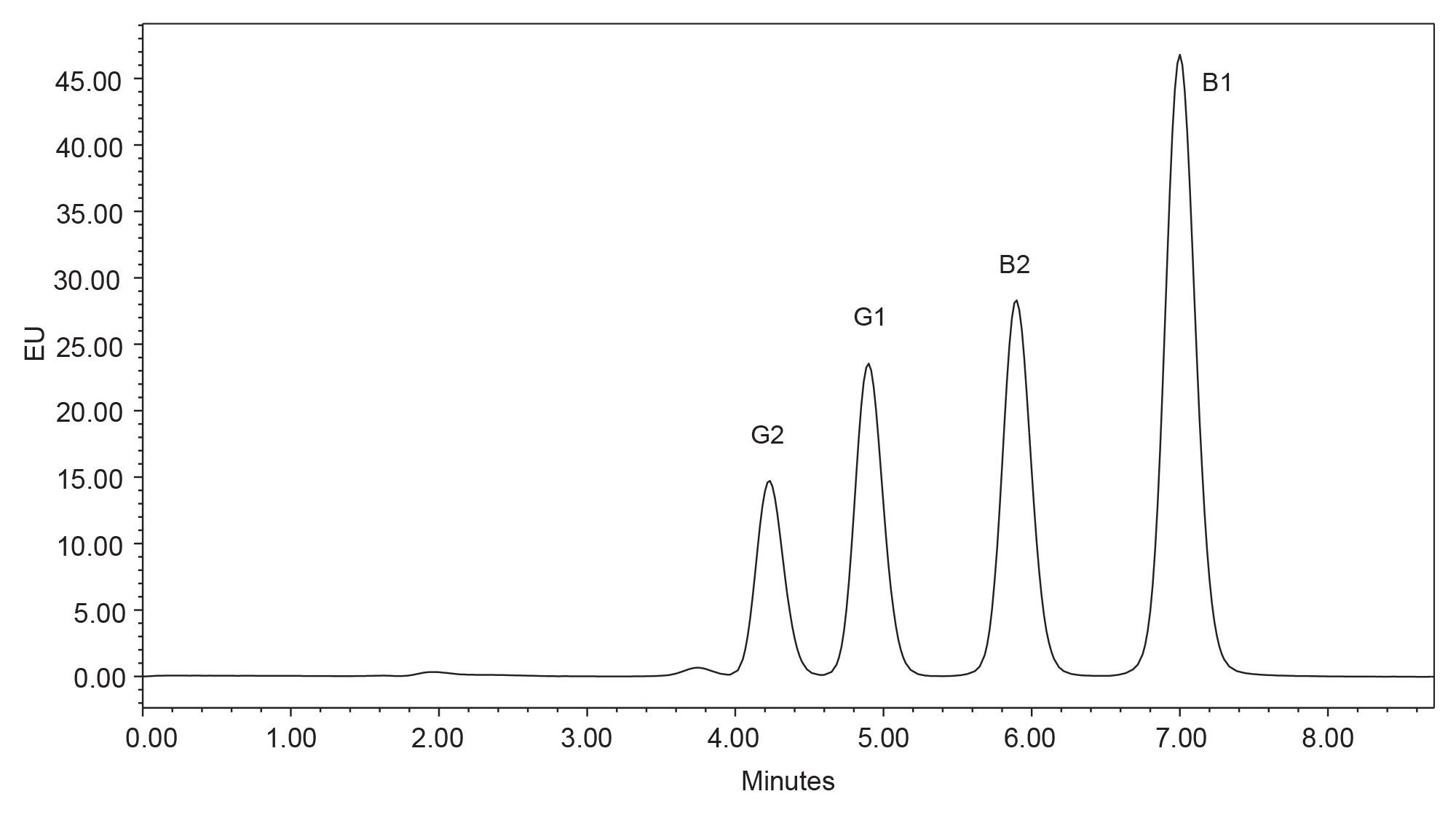
The original AOAC method 991.31 for aflatoxin analysis was transferred to the Arc HPLC using a photochemical reactor for derivatization of aflatoxins and updating the choice of column. Using a flow rate of 1 mL/min, aflatoxins were well separated (resolution >1.5), and peaks eluted between 6 and 11 min (Figure 2). However, as the pressure limit of the Arc HPLC is greater than older HPLC systems (now up to 9500 psi7), it is possible to increase the flow rate without reaching the pressure limit. If the flow rate is increased to 1.5 mL/min, the aflatoxin peaks eluted earlier without significant loss of resolution (resolution >1.5) and the run time can be shortened (Figure 3).
Limit of Detection and Quantification
Due to the low regulatory limits in food and low inherent fluorescence of aflatoxins B1 and G1, aflatoxin analysis can be enhanced using post-column derivatization, achieved using a photochemical reactor (PhCR) system. Using this configuration, the limit of detection of aflatoxin B1, B2, G1, and G2 at the flow rate of 1 mL/min was equivalent to 0.01, 0.006, 0.02 and 0.008 µg/kg in the sample, respectively; whilst the limit of quantification of aflatoxin B1, B2, G1, and G2 was equivalent to 0.03, 0.02, 0.05 and 0.02 µg/kg in the sample, respectively. Alternatively, analysis can be carried out with the photochemical detector omitted from the system. Figure 4 shows the relative response of the aflatoxins without post-column derivatization. Without the PhCR, the limit of detection for aflatoxin B1, B2, G1, or G2 at the flow rate of 1 mL/min was equivalent to 0.10, 0.005, 0.12 and 0.005 µg/kg in the sample, respectively.
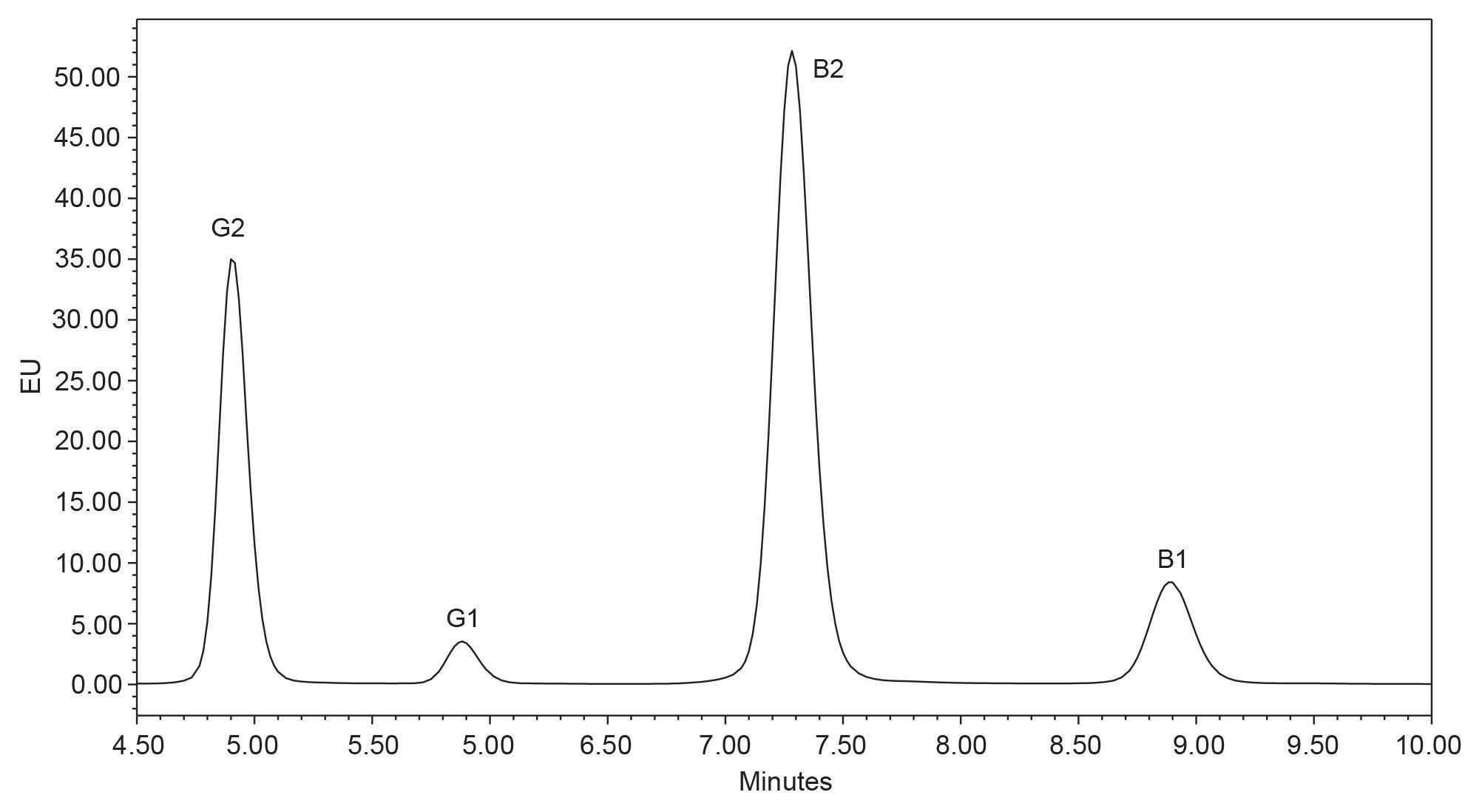
In the AOAC official method 2000.16 for the analysis of aflatoxin B1 in baby food using immunoaffinity column and HPLC, the detection limit of aflatoxin B1 in baby food is 0.1 µg/kg.10 Also, the European Union set the maximum detection level of aflatoxin B1 as 0.1 µg/kg in processed cereal-based foods and baby food for infants and young children, as well as dietary foods for special medical purposes specially for infants.11 In the EU, the maximum level of total B1, B2, G1, and G2 in peanuts, tree nuts and some other oilseeds is 4 µg/kg. The herein described method using post-column derivatization presents limits of quantification significantly lower than 0.1 µg/kg and meets the requirements for analyzing aflatoxins in baby foods.
Linearity
The linear range of the method was in the range of 0.5–300 µg/kg (total aflatoxins) in the original sample. The linearity of the standard curve is determined by the determination coefficient (r2) from the calibration curve regression fit, which was >0.99 for all compounds.
There are different maximum levels of aflatoxins set by the regulations. For example, the maximum level of aflatoxin B1 in the regulated foods set by the European Union ranges between 0.1 and 12 µg/kg, the maximum level of total aflatoxins in the regulated foods set by European Union ranges between 4 and 15 µg/kg.11 The maximum total aflatoxin level in food set by the United States Food and Drug Administration (FDA) needs to be less than 20 µg/kg and the maximum total aflatoxin level in different feed is 20, 100, 200 or 300 µg/kg.12,13 These aflatoxin values all fall within the linearity range of the standard curves of aflatoxins in this method.
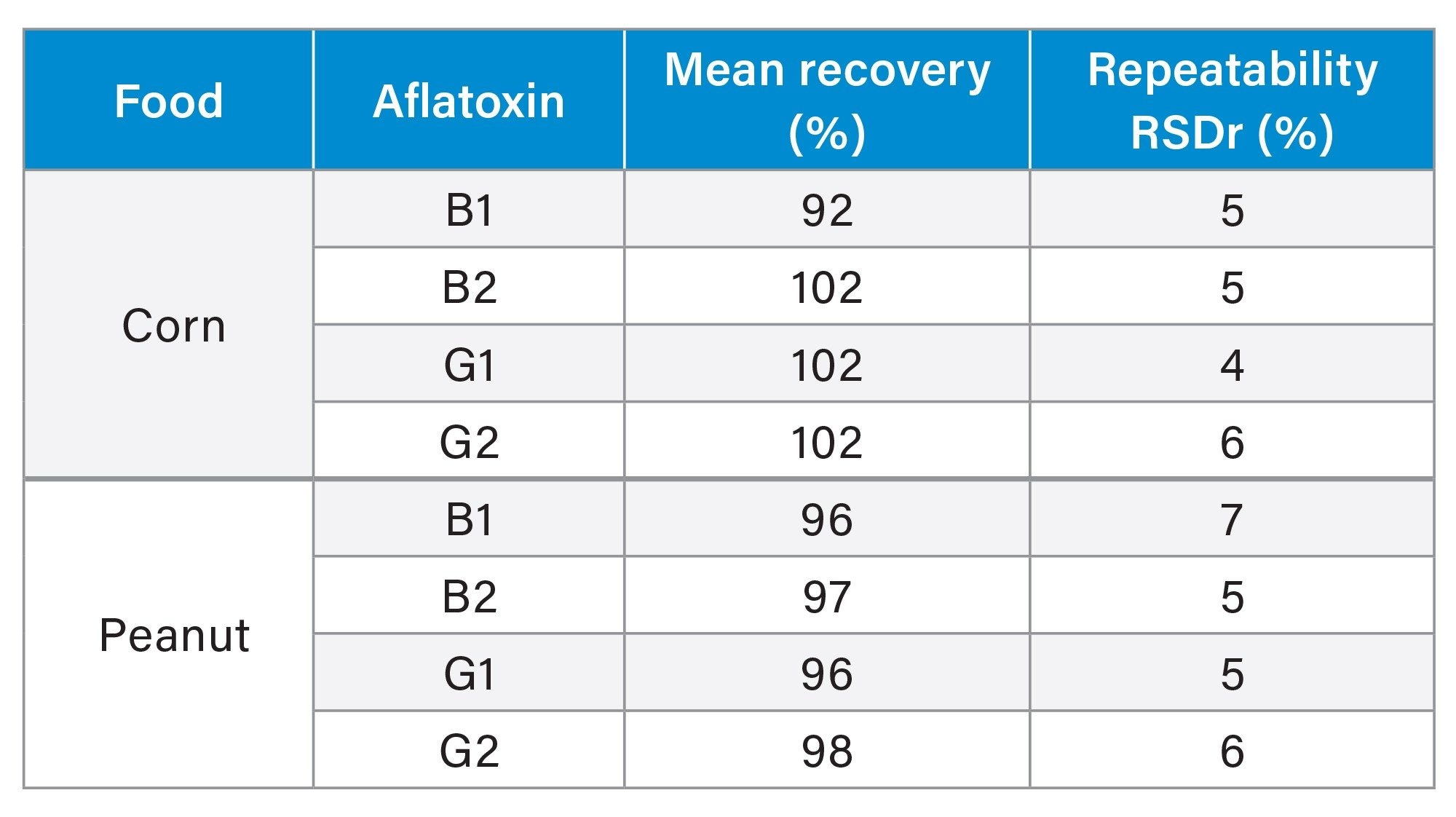
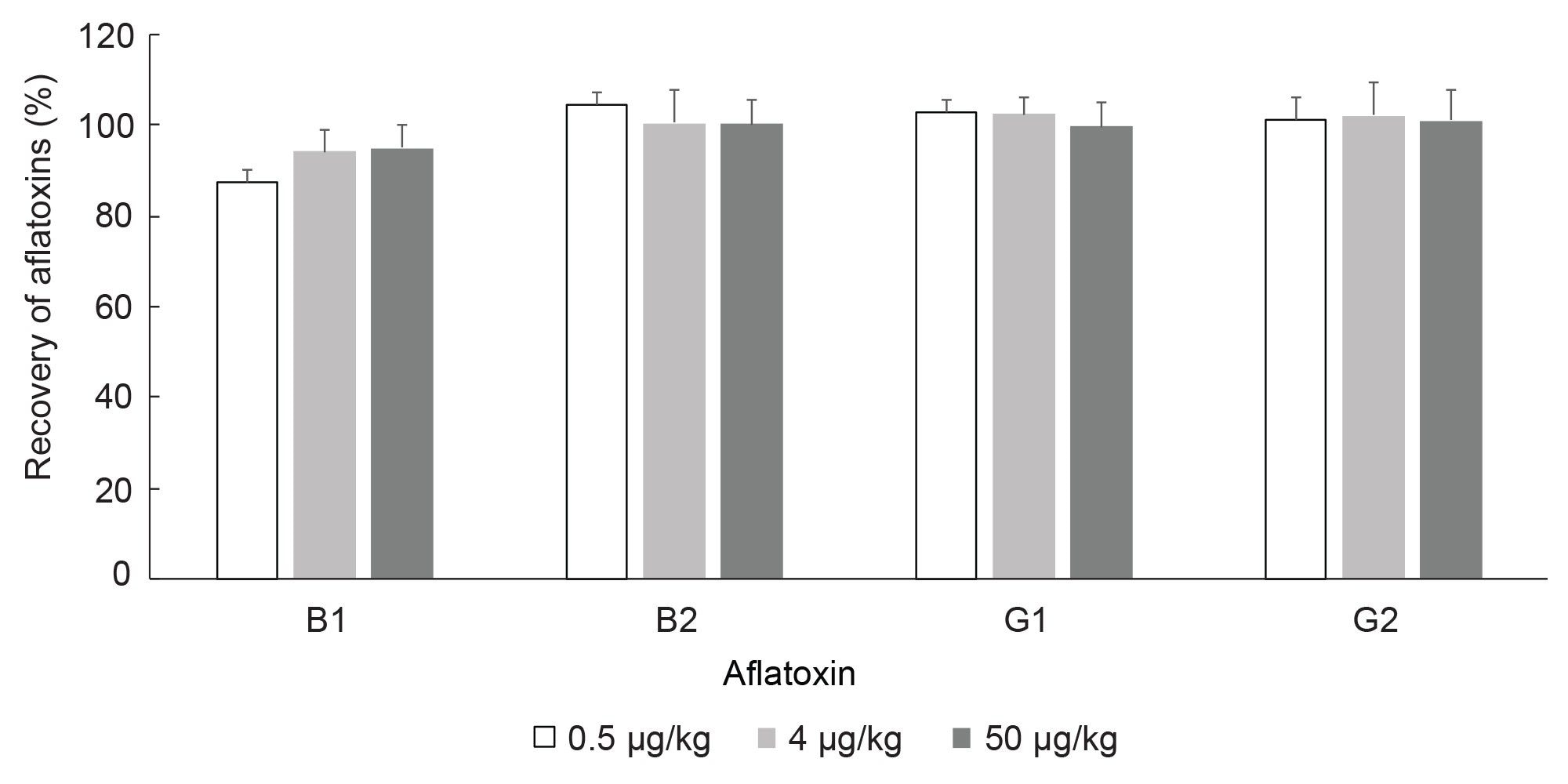
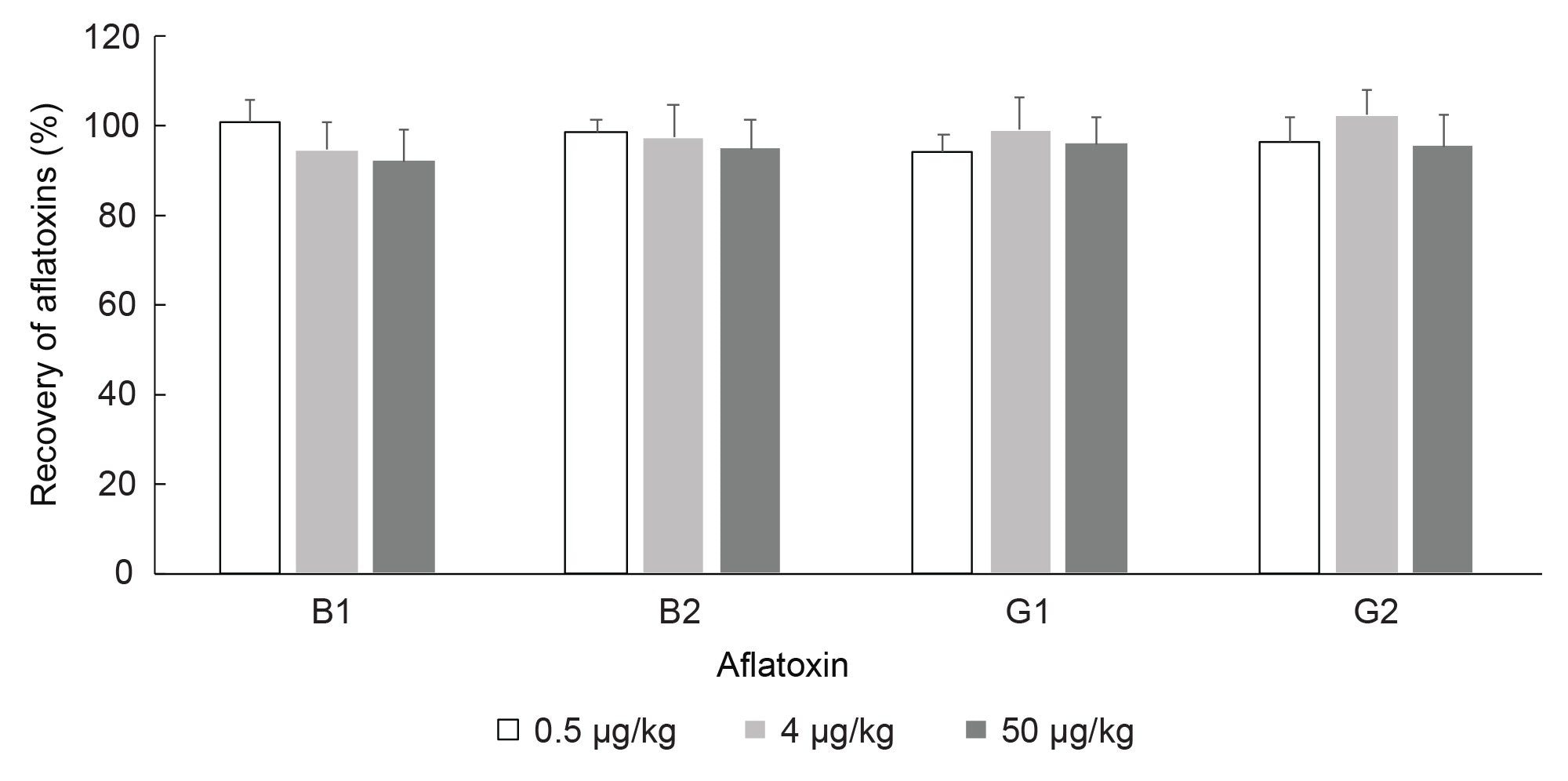
Recovery of Aflatoxins and Repeatability
Values for recovery of aflatoxins from analysis of corn or peanuts are shown in Table 1 and Figures 5 and 6. There were no detected aflatoxins in blank corn or blank peanuts. The mean values for recovery of aflatoxin B1, B2, G1, and G2 in corn or peanuts, at three spiking levels, was greater than 90%. Even though there are some variations in recovery, either between corn and peanut or among different spike levels, the recovery of aflatoxin B1, B2, G1, or G2 does not differ significantly between the two types of foods or among the three spike levels (Two-Way ANOVA, N=18, P >0.05). Overall, recovery of aflatoxin was more than 90% and standard deviation of recovery (n=9) was less than or equal to 7% (Table 1) for all aflatoxins and across all spiking levels. The recovery of aflatoxin in this work meets the recommended values provided by the European Commission (recovery of 50 to 120% for total aflatoxin <1.0 µg/kg, 70 to 110% for total aflatoxin 1–10 µg/kg and 80 to 110% for total aflatoxin >10 µg/kg).14
Conclusion
The AOAC method for aflatoxin analysis has been transferred to the Arc HPLC successfully. The method was improved by using a 3.5 µm particle size column, with increased chromatographic efficiency and reducing run time. The Arc HPLC copes well with the higher backpressure from the smaller particle size column. In addition, this work demonstrated that it is possible to reduce the volume of extract for immunoaffinity column chromatography (AflaTest WB), decreasing the time required for cleanup whilst maintaining sufficient sensitivity. The new method provided good detection and quantification limits, suitable linearity range, good recovery, and low standard deviation of recovery, all required to meet regulatory requirements for analysis of aflatoxins in food or feed, including baby foods.
References
- Abrar M, Anjum FM, Butt MS, Pasha I, Randhawa MA, Saeed F, Waqas K. Aflatoxins: Biosynthesis, Occurrence, Toxicity, and Remedies. Crit. Rev. Food Sci. Nutr. 2013 53(8):862–874.
- Mahato DK, Lee KE, Kamle M, Devi S, Dewangan KN, Kumar P, Kang SG. Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies. Front. Microbiol. 2019 10:2266.
- Singh J, Mehta A. Rapid and Sensitive Detection of Mycotoxins by Advanced and Emerging Analytical Methods: A Review. Food Sci. Nutr. 2020 8(5):2183-2204.
- Eskola M, Kos G, Elliott CT, Hajšlová J, Mayar S, Krska R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%, Crit. Rev. Food Sci. Nutr. 2020 60(16):2773–2789.
- Kaale LD, Kimanya ME, Macha IJ, Mlalila N. Aflatoxin Contamination and Recommendations to Improve its Control: A Review. World Mycotoxin J. 2021 14(1):27–40.
- Hird S, Martin K, Collette NZ, Toth D. Determination of Aflatoxins in a Wide Range of Food and Agricultural Commodities Using Immunoaffinity Chromatography Column Clean-up Coupled with UPLC or HPLC with Fluorescence Detection. Waters Application Note, 720007280, 2021 Jun.
- Arc HPLC System Instrument Specifications. 720006861, 2021 Nov.
- Waters Arc HPLC. 720006940, 2021 Feb.
- AOAC, Aflatoxins in Corn, Raw Peanuts, and Peanut Butter, Immunoaffinity Column (Aflatest) Method 991.31. AOAC INTERNATIONAL 2005.
- AOAC, Aflatoxin B1 in Baby Food Immunoaffinity Column HPLC Method 2000.16. AOAC INTERNATIONAL 2005.
- European Commission Regulation (EU) No 165/2010 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Aflatoxins. OJ L 50, 27.2.2010, p. 8–12.
- Sec. 555.400 Aflatoxins in Human Food. Food and Drug Administration: Rockville, MD. 2019.
- Sec. 683.100 Action Levels for Aflatoxins in Animal Food. Food and Drug Administration: Rockville, MD. 2019.
- European Commission Regulation (EC) No 401/2006 of 23 February 2006 Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs. OJ L 70, 9.3.2006, p. 12–34.
Acknowledgments
Ting Sun and Nancy Collette are affiliated with Waters | VICAM
720007843, January 2023