Size Exclusion Chromatography Method Migration Between ACQUITY™ Premier and Arc™ Premier Systems
This is an Application Brief and does not contain a detailed Experimental section.
Abstract
This application brief demonstrates Size Exclusion Chromatography (SEC) method migration for monoclonal antibody analysis between ACQUITY Premier and Arc Premier systems with the use of XBridge™ Premier Protein SEC 250Å and ACQUITY Premier Protein SEC 250Å Columns.
Benefits
- Waters™ MaxPeak™ High Performance Surfaces (HPS) Technology specifically engineered for protein SEC separations minimize secondary interactions for increased consistency of SEC results
- MaxPeak Premier columns are available in a variety of formats and particle sizes to facilitate method migration between HPLC, UHPLC, and UPLC™ platforms
Introduction
SEC is a well-established technique for separating biotherapeutics based on their hydrodynamic volume.1 These biotherapeutics are generally protein-based and are monitored for aggregation and fragmentation impurities that affect product efficacy and safety. The largest challenges associated with analyzing these impurities are the ionic and hydrophobic secondary interactions that can occur between the proteins and the particle or hardware surfaces. To address this, Waters has developed MaxPeak HPS Technology for SEC particle and hardware surfaces that reduce undesired secondary interactions and maximize recovery and resolution. As part of this offering, Waters has incorporated a hydroxy terminated polyethylene oxide bonded particle into the BEH particle surface (BEH-PEO) as well as engineered the column hardware to be more inert towards analytes to provide a new level of separation efficiency to SEC.2 These newly engineered MaxPeak Premier SEC Columns offer users the ability to analyze biotherapeutics with minimal to no salt or organic solvent additives allowing for reliable quantitation of protein size variants with simple biorelevant buffers.
Furthermore, Waters offers the MaxPeak Premier SEC Columns in a variety of particle sizes and formats, allowing customers to take full advantage of the performance offered by the MaxPeak Premier SEC Columns to support their analytical needs across pharmaceutical development and commercialization. To demonstrate this, a SEC method developed on the ACQUITY Premier System using a 1.7 µm ACQUITY Premier Protein SEC 250Å Column was migrated to an Arc Premier System using a 2.5 µm XBridge Premier Protein SEC 250Å Column.
Results and Discussion
SEC is the gold standard method for separating and quantifying size variants for the development and quality control of monoclonal antibodies (mAbs). In this study, mAb therapeutics Remicade™ and an approved biosimilar, Renflexis™, (post-expiration) were analyzed for size variants on both the ACQUITY Premier UPLC System and Arc Premier UHPLC System. SEC method conditions were developed on the ACQUITY Premier System using an isocratic gradient with sterile filtered 1X PBS, pH 7.4 and an ACQUITY Premier Protein SEC 250Å Column (4.6 mm x 300 mm, 1.7 µm). Even with the utilization of an isocratic gradient, parameters such as flow rate and injection volume must be scaled between systems to maintain the same column loading and column volumes for migration of chromatographic performance. These method conditions were scaled using the Waters Column Calculator version 2.0 and migrated to an Arc Premier System with a XBridge Premier Protein SEC 250Å Column (7.8 mm x 300 mm, 2.5 µm). The equivalent separations of innovator and biosimilar mAbs are displayed in Figures 1 and 2.
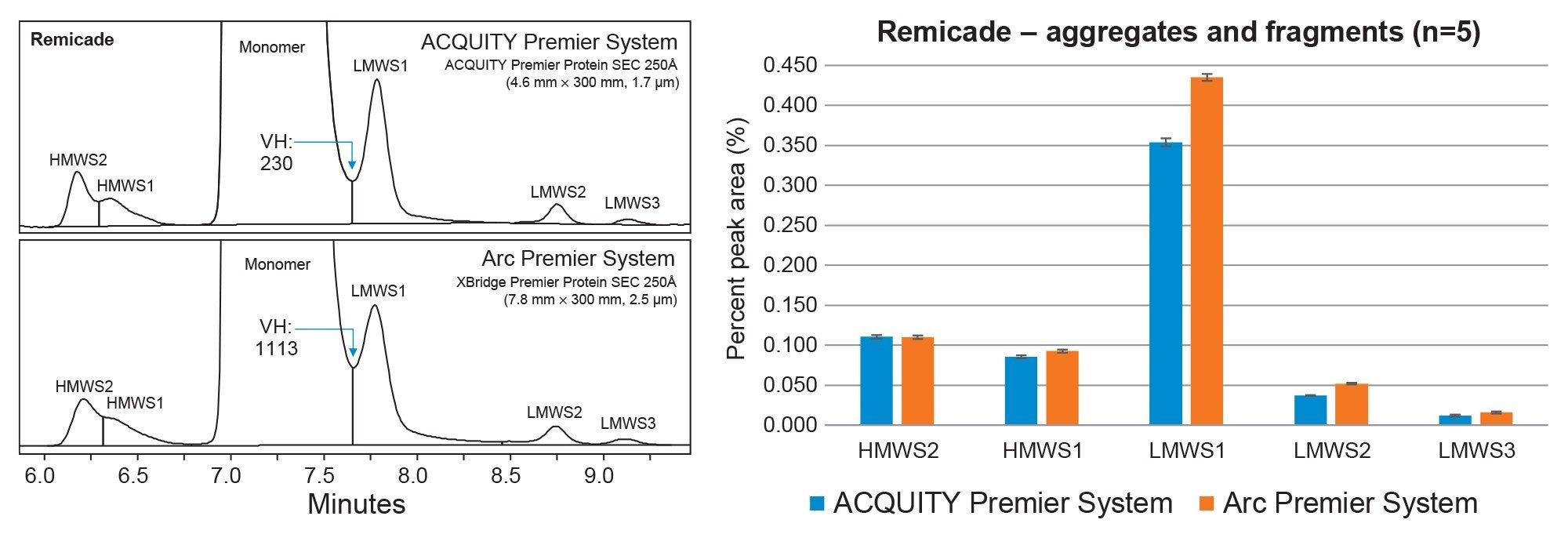
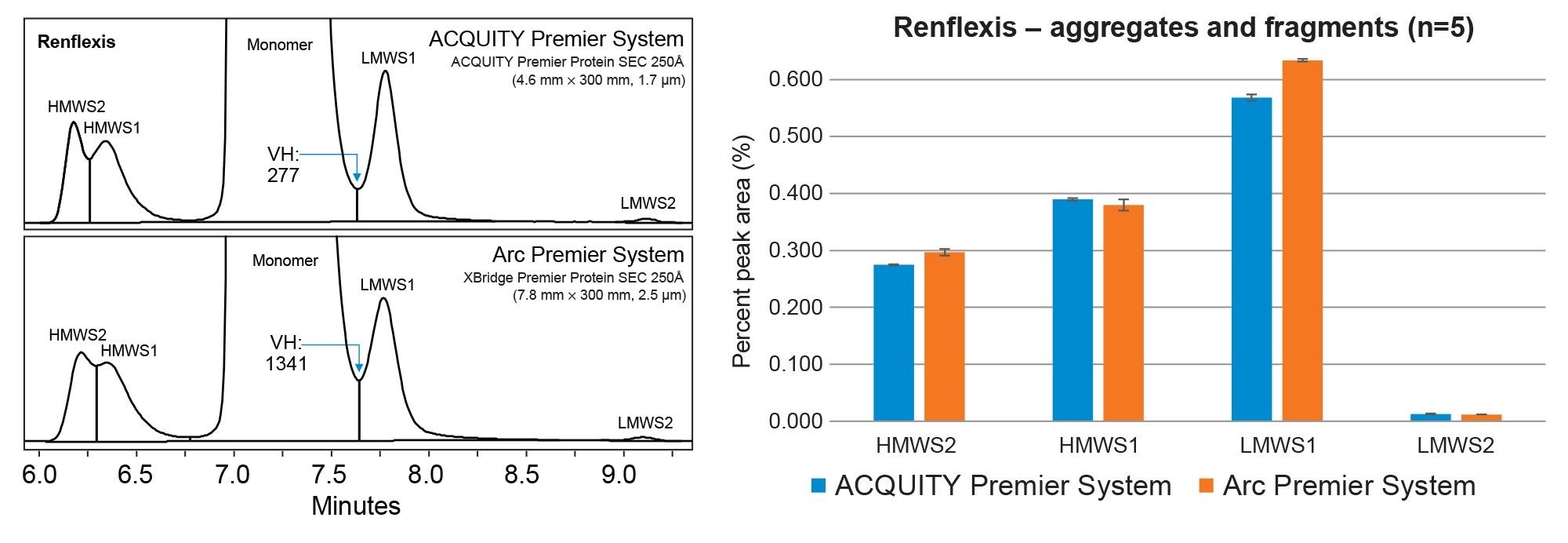
The relative percent peak area was averaged for five replicate injections of Remicade and Renflexis to compare chromatographic performance between the original UPLC (ACQUITY Premier System) and scaled UHPLC method (Arc Premier System). The resolution between the monomer and LMWS1 was monitored by dividing the peak height and ratio of peak height to valley height to calculate the valley height (VH) of the fused peak using a custom field within the Empower CDS. As shown in the associated bar plot for both mAbs, the relative percent peak area was within ~0.1% of the original method for aggregates and fragments. The ACQUITY Premier System was observed to have the lowest VH between peaks due to the improved resolution attributed to smaller particle size. While this observation had negligible impact in terms of overall peak area% and purity determinations between systems (Remicade: 99.4% vs 99.3%, Renflexis: 98.8% vs 98.7%), it could factor into design of experiment (DOE) considerations for other analytes. Alternatively, users can achieve an ~20% reduction in SEC analysis time on the ACQUITY Premier Protein SEC 250Å 1.7 µm Column by increasing the flowrate and matching the resolution obtained on the XBridge Premier Protein SEC 250Å 2.5 µm Column for increased throughput in their SEC analyses. Collectively, the data demonstrates that the MaxPeak Premier SEC Columns offers users the ability to resolve size related variants reproducibly across systems and column formats facilitating easier method migration across biopharmaceutical development, manufacturing, and quality organizations.
Conclusion
SEC is a powerful chromatographic technique for the characterization, process monitoring, and quality control release of biotherapeutics. Utilizing a variety of columns and chromatography systems with MaxPeak High Performance Surface Technology, methods that were developed during characterization were migrated downstream for process monitoring while maintaining robust and equivalent performance. This study demonstrated that two mAbs analyzed on an ACQUITY Premier System and Arc Premier System exhibited ~0.1% relative percent peak area difference for aggregates, fragments, and the monomer. This consistency confirmed that SEC methods can be successfully migrated across LC platforms using the Waters Column Calculator and MaxPeak Premier HPS enabled particle and hardware surfaces.
References
- Yang H, Koza SM, Yu YQ. Determination of Hydrodynamic Radius With MaxPeak Premier Protein SEC Columns. Waters Application Note, 720007625 2022.
- Kizekai L, Shiner SJ, Lauber MA. Waters ACQUITY and XBridge Premier Protein SEC 250Å Columns: A New Benchmark in Inert SEC Column Design. Waters Application Note, 720007493 2022.
720007836, January 2023