This application note describes a streamlined UPLC System solution with PDA and single quadrupole MS (SQD) detectors for tea seed oil characterization, quality control, and authentication. This solution provides tea seed oil companies with an efficient and easy method to establish their quality standards and authenticate their products.
Tea seed oil is an edible oil cold-pressed from the seeds of Camellia oleifera and Camellia sinensis (Figure 1). It is mainly produced in Hunan, Jiangxi, Guangxi, and other southern provinces of China. In Chinese herbal medicine, tea seed oil is considered a superior nutritional dietary supplement that benefits the digestive system, reduces bad cholesterol, lowers blood pressure, regulates the nervous system, and strengthens the immune system.1,2 Tea seed oil is recommended by the Food and Agriculture Organization of the United Nations as a high-quality, healthy vegetable oil because of its nutritional value, which is comparable to olive oil in terms of its high oleic acid content, low saturated fat, high antioxidants, and excellent storage qualities.3

Tea seed trees are evergreen plants that can grow on barren land without fertilizers. They start bearing fruits eight years after initial planting, and can remain highly productive for 80 years. In an effort to create more green land, increase farmers’ income, and reduce China’s dependence on imported food, Chinese government agencies are setting policies to support the growth of the tea seed oil industry. At present, annual production of tea seed oil is approximately onequarter million tons and expected to reach more than three million tons by 2020 or 15–25% of the total edible oil supply in China.3
To satisfy legislative requirements worldwide,4 and establish the premium quality oil brand names, tea seed oil companies in China monitor the entire production process, from tree cultivation, harvesting and cold-pressing tea seeds, to oil packaging and shipping. As a result, analytical technologies that can streamline quality control and help to differentiate their products are in increasing demand. Currently, seed oil analysis mainly relies on GC and HPLC methods. GC methods require derivatization prior to analysis, which is timeconsuming and laborious.5 Conventional HPLC methods require either using halogenated solvent or using non-halogenated solvent with longer runtimes to achieve adequate separation.6–9 The use of halogenated solvents are restricted in many laboratories since they are known carcinogens and environmental hazards.
The Waters ACQUITY UPLC System is a new generation of liquid chromatographic platform. Using UPLC/UV photodiode array (PDA)/mass spectrometer detectors, fast screening and high resolution methods for seed oil characterization have been developed without using halogenated solvents.10–13
The ACQUITY UPLC System with PDA Detector enables the acquisition of multiple data types in a single injection to generate reproducible fingerprinting information, identify triglyceride components, and evaluate the degree of seed oil oxidation and decomposition. Compared with conventional HPLC, UPLC shortens analysis times, reduces solvent usage, and provides a higher resolution chromatogram with more information in a single injection. As a result, the UPLC method is more cost-effective.
This Application Note describes a streamlined UPLC System solution with PDA and single quadrupole MS (SQD) detectors for tea seed oil characterization, quality control, and authentication. This UPLC solution provides tea seed oil companies with an efficient andeasy method to establish their quality standards and authenticate their products. The note also compares the composition of tea seed oil with olive oil and other vegetable oils. Among the samples examined, tea seed oil exhibited the highest trioleoylglycerol content among all the oils. It contains very high omega-9 fatty acid content, but very low omega-6 and saturated fatty acid contents. This may be one of the reasons why tea seed oil has been recognized for health benefits since ancient times in Chinese medicine.
Commercial tea seed oil was received from China. Other edible oils were bought from local grocery stores. All oil samples were diluted with 2-propanol to make a 6 mg/mL solution for the analysis.
|
UPLC system: |
ACQUITY UPLC with PDA and SQ detectors |
|
Software: |
Empower 2 |
|
Column: |
ACQUITY UPLC BEH C18, 2.1 x 150 mm |
|
Column temp.: |
30 °C |
|
Weak wash solvent: |
2-propanol (500 μL per wash) |
|
Strong wash solvent: |
2-propanol (500 μL per wash) |
|
Seal Wash solvent: |
10% CH3CN in H2O (every 5 min) |
|
Mobile phase A: |
CH3CN |
|
Mobile phase B: |
2-propanol |
|
Injection: |
2 μL (full loop) |
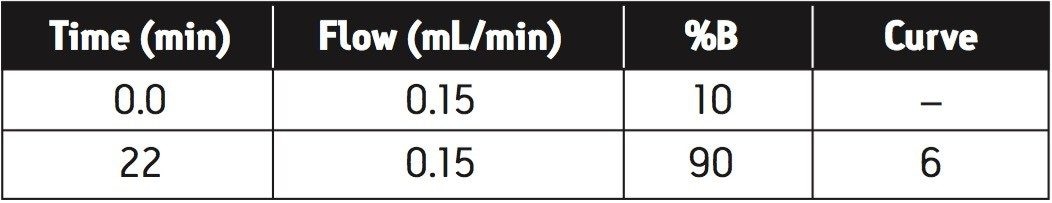
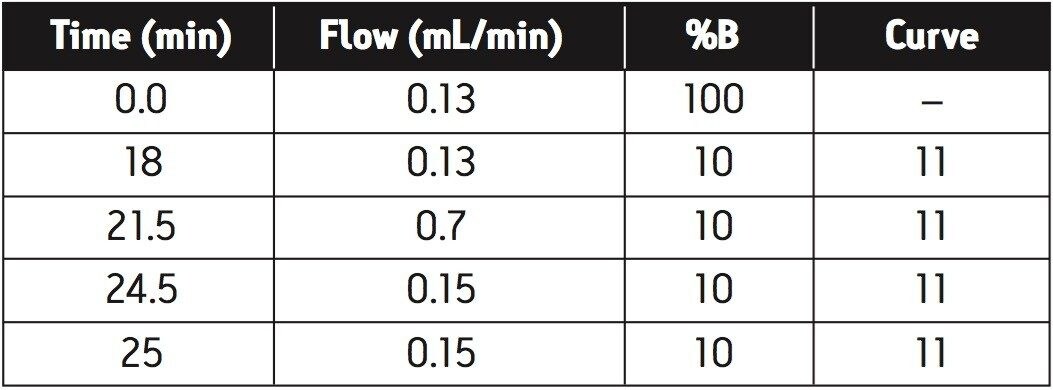
|
Detection: |
195 to 300 nm |
|
Sampling rate: |
20 pts/s |
|
Filter response: |
fast |
|
Instrument: |
ACQUITY SQD with IonSABRE APCI probe |
|
Ionization mode: |
APCI+ |
|
Corona (μA): |
5 |
|
Cone voltage: |
+30 V |
|
Extractor: |
+3 V |
|
Source temp.: |
150 °C |
|
APCI Probe temp.: |
450 °C |
|
Desolvation gas: |
700 L/hr |
|
Cone gas: |
0 L/hr |
|
Acquisition range: |
140 to 1100 m/z |
It is difficult to separate triglycerides, the major components of seed oil, using conventional HPLC methods without halogenated solvents. The ACQUITY UPLC System includes running high-efficiency columns packed with small particles to perform faster, sensitive, well-resolved separations. The UPLC solvent delivery system can sustain back pressures up to 15,000 psi, enabling the use of high viscosity solvent such as 2-propanol for seed oil analysis. Since 2-propanol is good for dissolving seed oil,14 low in toxicity, and allows UV detection of triglycerides due to its low limit of transparency; 2-propanol was chosen as the strong eluent. Unlike other solvents used in conventional HPLC methods, acetonitrile and 2-propanol used in UPLC methods are compatible with PDA and MS detectors for seed oil analysis. Multiple data types can be obtained in a single injection to generate reproducible fingerprinting data,10,13 identify triglyceride components by mass spectrometry, and evaluate the degree of seed oil oxidation with multiple PDA wavelength channels.11
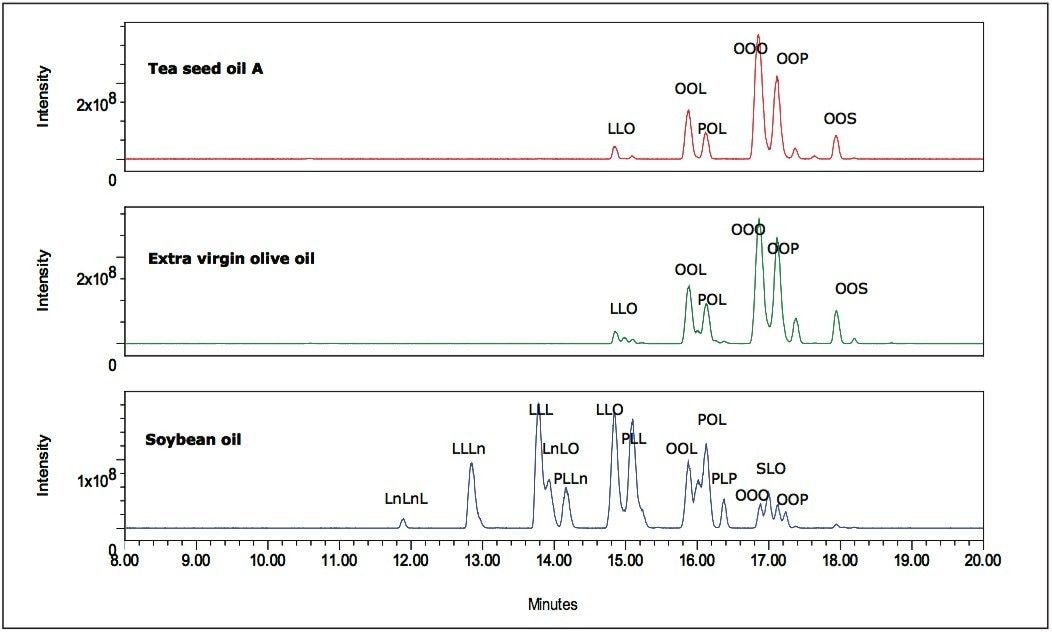
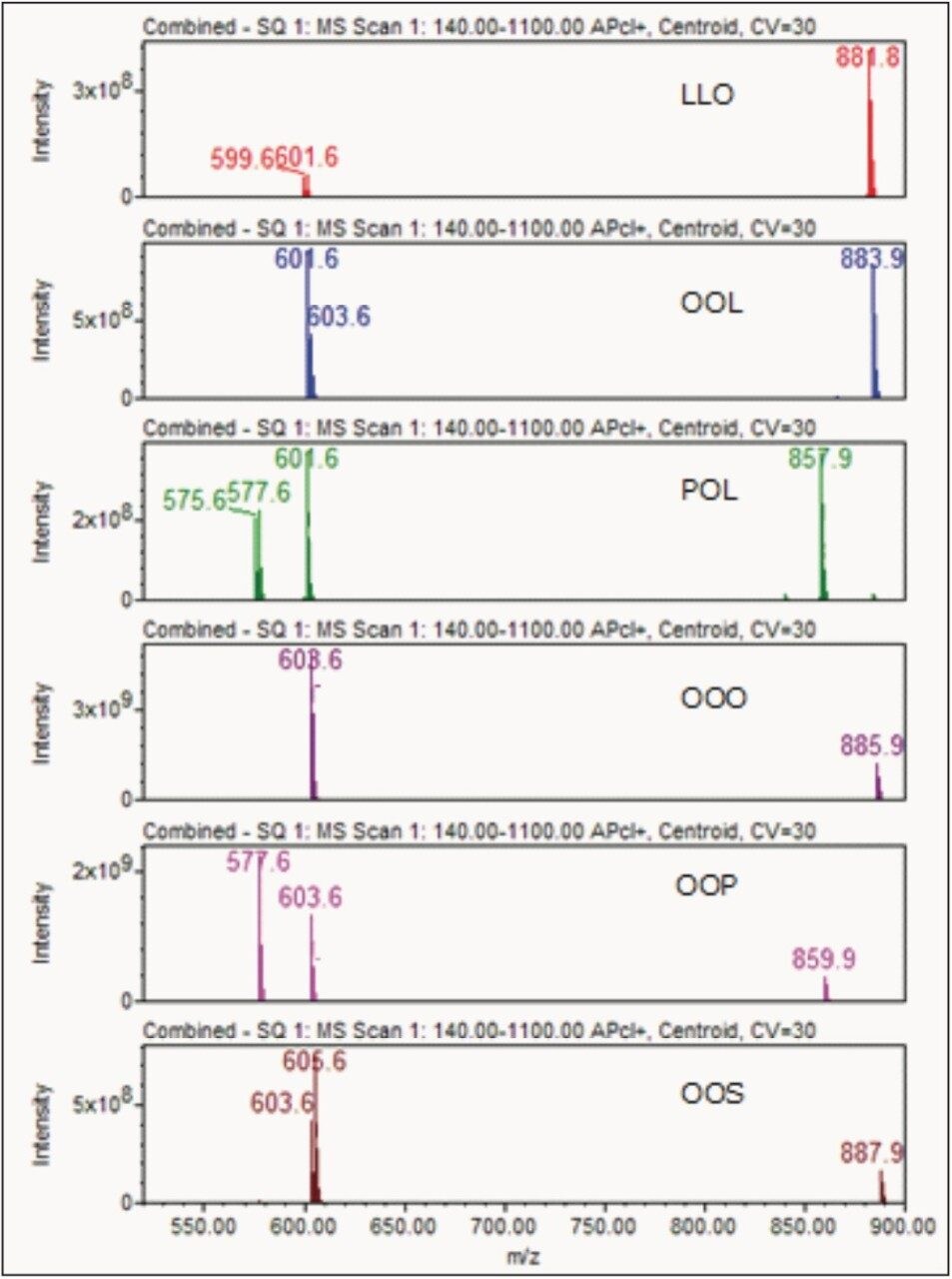
Figure 2 shows positive APCI TIC chromatograms of tea seed oil A, extra virgin olive oil, and soybean oil samples. In comparison, chromatograms of tea seed oil and olive oil show similar triglyceride peak patterns, but chromatogram patterns of tea seed oil and soybean oil are dramatically different. The triglyceride components can be identified by a well-established tandem mass spectrometry method according to observed pseudomolecular ion and relative intensity of diacylglycerol fragment ions.15,16 Since triglycerides are well separated by UPLC, they can be identified using the ACQUITY single quadrupole mass detector (SQD). As shown in combined mass spectra of the major peaks of tea seed oil (Figure 3), using observed pseudomolecular ion and diacylglycerol fragment ions, the major components of tea seed oil are identified as follows:
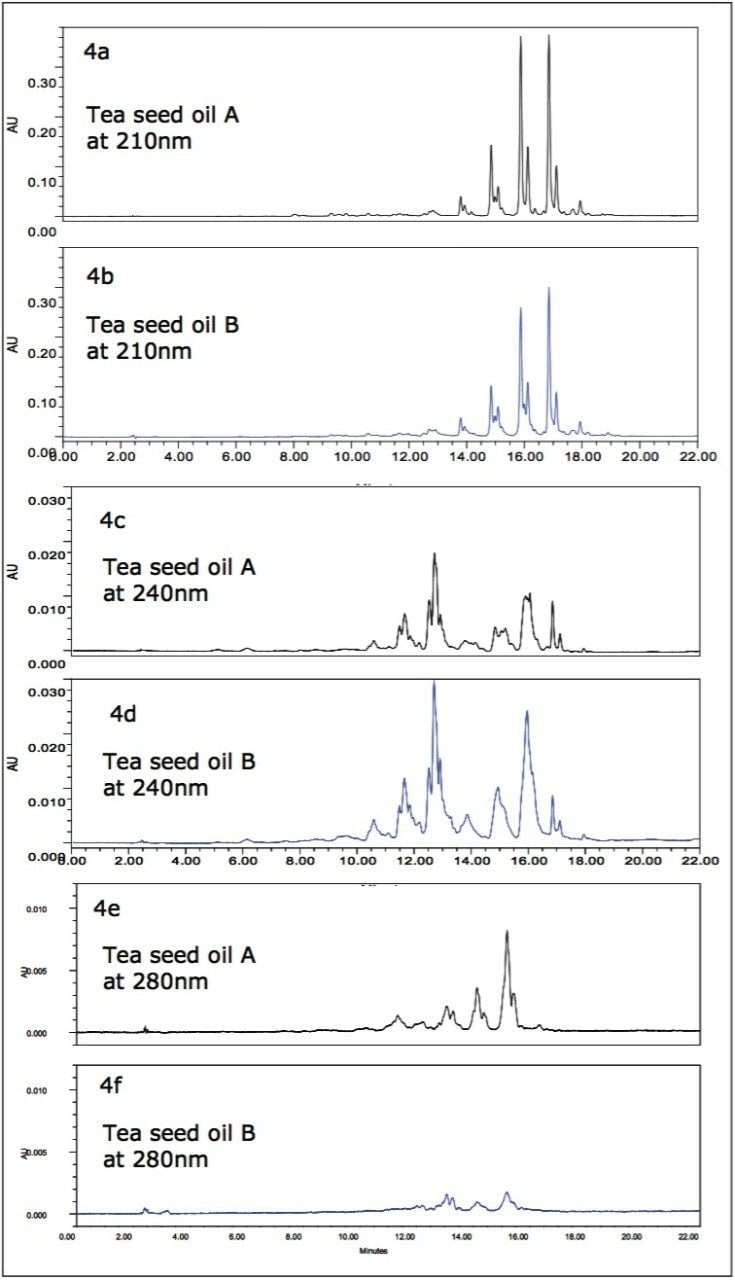
Oxidation of triglycerides, known as rancidification, occurs upon exposing triglycerides to air. Most seed oil companies make great efforts to prevent seed oil oxidation during production processes, packaging, and storage. The degree of oxidation of seed oil samples can be monitored using the ACQUITY UPLC System with PDA Detector. While unspoiled triglycerides have UV absorption at about 210 nm, oxidized triglycerides containing conjugated diene, aldehyde, ketone, or carboxylic acid functional groups have UV adsorption at higher wavelength. Figures 4a to 4f show UV PDA extracted chromatograms at 210, 240, and 280 nm wavelengths of two different brands of tea seed oil samples from different provinces in China. The 210 nm chromatograms of tea seed oil A and B have similar triglyceride peaks but different relative intensities, as shown in Figures 4a and 4b. The difference in triglyceride component ratios between the two brands of tea seed oil samples may due to the variation of tea trees, geography, or production conditions. The 240 nm chromatogram of tea seed oil B has more high intensity peaks than that of tea seed oil A, as shown in Figures 4c and 4d, which indicate more oxidized triglycerides with conjugated dienes in tea seed oil B relative to tea seed oil A.18 Interestingly, the 280 nm chromatogram of tea seed oil B has much lower intensity peaks than tea seed oil A (Figure 4e and 4f), indicating that tea seed oil A contains more oxidized triglycerides with conjugated aldehyde or ketone functional groups. Clearly, the two tea seed oil samples are in different stages of oxidation.
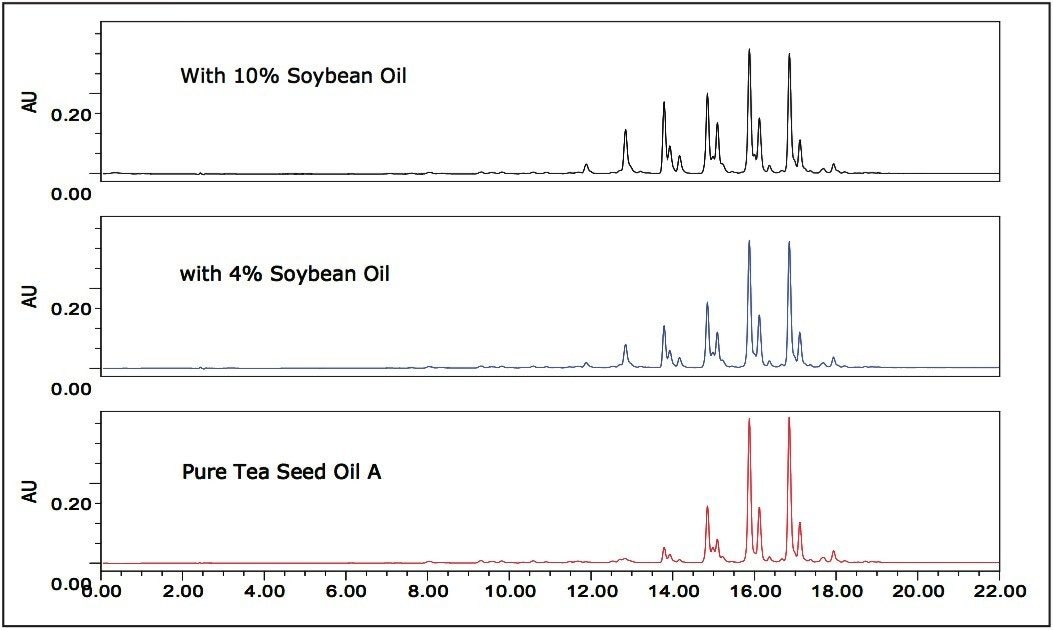
It is known that seed oils have characteristic ratios of triglycerides useful for seed oil identification, quality control, and authentication.8,9,13 Expensive tea seed oil, like extra virgin olive oil, can be adulterated with less expensive oils such as soybean oil. It is critical for tea seed oil companies to be able to safeguard their products against counterfeit goods. Figure 5 shows 210 nm extracted chromatograms of pure tea seed oil A, tea seed oil A samples adulterated with 4% and 10% soybean oil respectively. The adulterated samples can be easily recognized by the change of relative peak intensity, especially those peaks at retention times between 11.0 to 14.5 min. Seed oil producers can use UPLC-PDA generated, highly reproducible fingerprinting chromatograms to differentiate and authenticate their products according to characteristic ratios of triglyceride peaks.13 The custom field calculation functions in Empower 2 Software can be utilized to automatically convert raw chromatographic data into a “Pass” or “Fail” report based on userset triglyceride peak ratio QC criteria.13 These advanced functions of Empower 2 eliminate the need for manual calculations, and help, prevent potential human errors by delivering critical information with speed and accuracy.
The ACQUITY UPLC-PDA-SQD System with Empower 2 Software enables rapid analysis and authentication of tea seed oil samples without derivatization and halogenated solvents. Multiple types of data are obtained in a single injection to generate well-resolved and reproducible data for tea seed oil authentication, triglyceride identification, and seed oil oxidation evaluation. The UPLC solution enables tea seed oil producers to quickly evaluate the stages of seed oil oxidation during extraction, packaging and storage to ensure product quality. Seed oil companies can use the highly reproducible fingerprinting chromatograms with great ease and confidence to differentiate their products from counterfeit goods and protect their brand names. The UPLC System shortens analysis time, reduces solvent usage, and provides more information, resulting in great cost benefit.
Tea seed oil has high healthy omega-9 fatty acid content but very low omega-6 content and saturated fatty acid content. This may help explain why tea seed oil has been used in Chinese medicine to benefit human health.
720002980, March 2009