This is an Application Brief and does not contain a detailed Experimental section.
USP monograph for budesonide was followed to successfully separate the two epimers on the Alliance HPLC System using a software tool to select a new Waters column chemistry.
The Alliance HPLC System is ideally suited for quality control laboratories that require a robust and reliable HPLC system for monitoring the quality of pharmaceutical products.
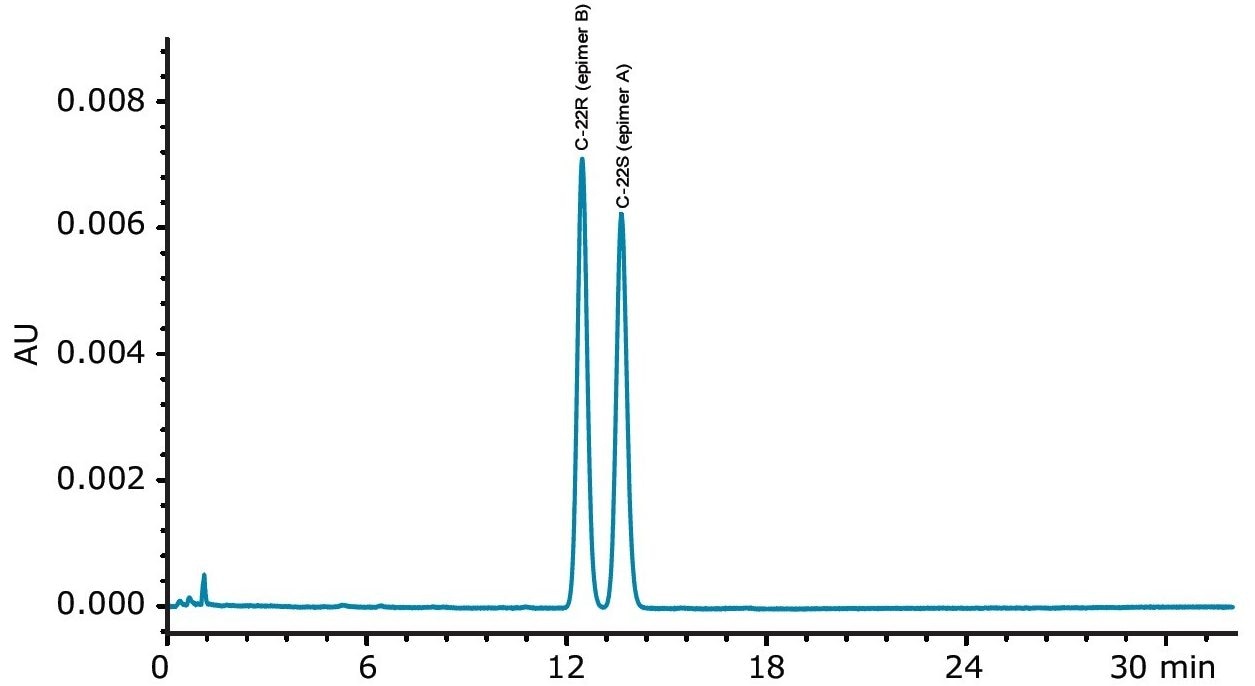
Budesonide is a corticosteroid used for the treatment of asthma, non-infectious rhinitis (including hay fever and other allergies), and treatment and prevention of nasal polyposis. Additionally, it is used for inflammatory bowel disease. Budesonide (epimers of 16α,17-[(1RS)-butylidenebis(oxy)]-11β,21-dihydroxypregna-1,4-diene-3,20-dione) is a mixture of the C-22S (epimer A) and the C-22R (epimer B).
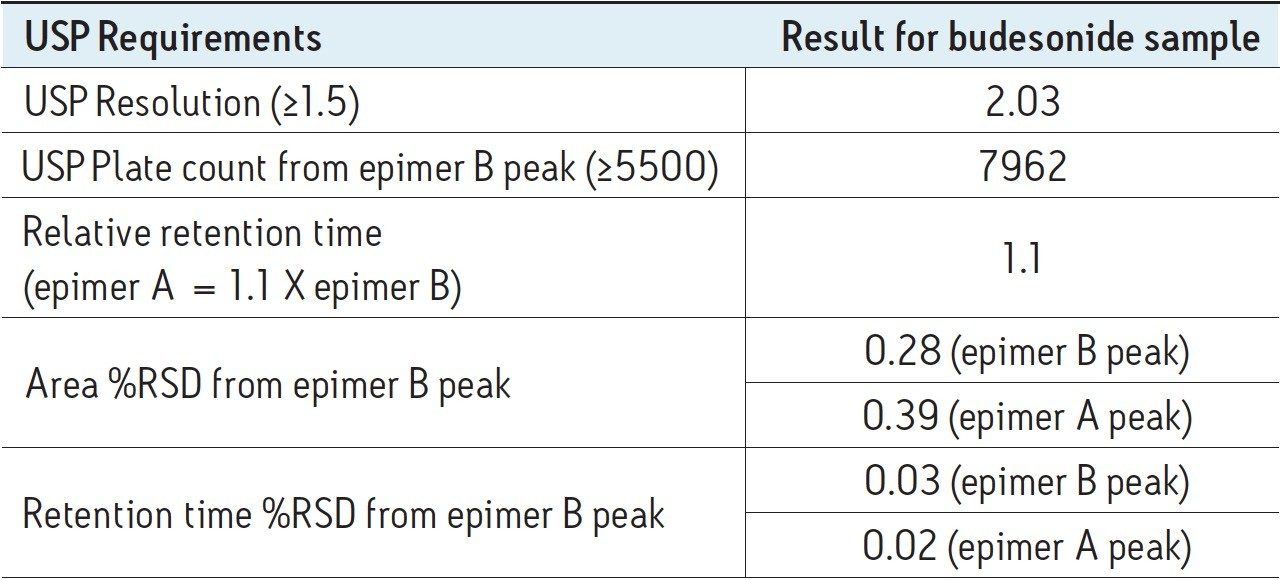
The USP monograph for budesonide requires the use of a 4.6 x 15 cm, 5 µm packing L1 column to be run on an HPLC system with UV detection at 254 nm and 23 mM phosphate buffer with acetonitrile at pH 3.2. The USP monograph has three system suitability requirements. The resolution between the two budesonide epimer peaks is not less than 1.5. The column efficiency is not less than 5500 theoretical plates, determined from the budesonide epimer B peak. The relative retention time for epimer A is 1.1, with respect to epimer B. Such a sensitive USP assay run in a typical quality control laboratory would require the use of a reliable and robust HPLC system capable of accurate and precise pumping, stable mixing, and accurate sample injection and detection. The Alliance HPLC System delivers this high level of performance, as demonstrated in this study.
The Alliance HPLC System, as shown in Figure 1, is suited for quality control laboratories that require a robust and reliable HPLC system for monitoring the quality of pharmaceutical products. The original methodology in the USP monograph was developed and submitted using an older L1 C18 column technology. The Waters Reversed Phase Column Selectivity Chart was used to find a similar but more modern and robust L1 column that offers the closest match in retentivity and selectivity. The Waters XBridge C18 column was selected. The concentration of the working standard and formulation sample specified in the monograph was 0.5 mg/mL. This specified concentration was for the API but was not appropriate for the final formulation, as it contained the API suspended in a mix of excipients. Therefore, the sample preparation was amended in this experiment to prepare the standard and the sample concentration at 12.8 µg/mL to ensure the formulation preparation was sufficiently dissolved.

The separation of the budesonide standard was run using the XBridge C18 4.6 x 150 mm, 5 μm (L1) Column, as shown in Figure 2.
As seen in Table 1, system suitability requirements for the budesonide separation on the Alliance HPLC System were easily met. In addition, the area and retention time repeatability values for both epimer B and A peaks are substantially better than the USP requirement of 2.0% RSD for five replicate injections as specified in USP Chromatography Chapter 621. The Alliance HPLC System is, thus, capable of delivering very precise chromatographic results.
USP monograph for budesonide was followed to successfully separate the two epimers on the Alliance HPLC System using a software tool to select a new Waters column chemistry. System suitability requirements for the USP method were met. The performance characteristics of the Alliance HPLC System resulting in delivery of repeatable and reliable chromatographic results are suited for successfully running USP compendial methods.
720004539, January 2013