This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the enhanced resolving power of the ACQUITY UPLC Glycoprotein BEH amide, 300Å, Column for separations of high molecular weight, RapiFluor-MS labeled N-glycans.
Wide-pore glycoprotein BEH amide, 300Å, 1.7 μm columns for enhancing the resolution of tri- and tetra-antennary, RapiFluor-MS labeled N-glycans.
Protein glycosylation is frequently profiled by removing glycans from their counterpart glycoprotein and imparting them with a detectable chemical moiety, such as the fluorescence and MS-active RapiFluor-MS label.1 High resolution separations of these released and labeled N-glycans can be obtained by UPLC hydrophilic interaction chromatography (HILIC) with purposefully designed glycan BEH amide, 130Å columns.2 Interestingly, glycosylation of proteins can be extremely diverse. While monoclonal antibodies tend to be modified with relatively low molecular weight (1 to 3 kDa) biantennary structures, numerous biotherapeutic proteins are expressed with comparatively high molecular weight (3 to 6 kDa) tri- and tetra-antennary structures. Such large and highly branched glycan structures exhibit large radii of hydration.
Consequently, the application of chromatography columns containing particles with standard average pore diameters (80 to 150Å) can limit the resolution with which these species can be separated. It is therefore advantageous to employ a stationary phase with a wide average pore diameter, wherein large structures will have access to the majority of the porous network and the surface area of the stationary phase. In addition, the large labeled glycan structures are less likely to experience restricted diffusion while migrating through the pores of a widepore material.3-4 In this technology brief, we demonstrate the utility of an amide bonded stationary phase with an average pore diameter of 300Å (glycoprotein BEH amide, 300Å, 1.7 μm) and its ability to enhance the resolution of RapiFluor-MS labeled tri and tetra-antennary N-glycans derived from recombinant human Factor IX.
N-glycans were prepared from both pooled human IgG as well as recombinant human Factor IX, a glycoprotein known to be modified with large, highly sialylated N-glycans.5 Specifically, these samples were prepared using rapid deglycosylation, RapiFluor-MS labeling, and μElution HILIC SPE, as described in the GlycoWorks RapiFluor-MS N-Glycan Kit Care and Use Manual.1, 6 Glycan mapping of the resulting RapiFluor-MS labeled N-glycans was first performed with a glycan BEH amide, 130Å, 1.7 μm column, given that it is intended for general purpose glycan analyses and for use with GlycoBase database searching.7 Figure 1A displays the fluorescence chromatograms obtained when HILIC-based chromatography is performed on a sample containing less hydrophilic IgG N-glycans (orange) versus a sample containing later eluting, more hydrophilic N-glycans from Factor IX (blue). In addition, Figure 1A displays the effective peak capacities for the retention windows of the two sample types. These results can be compared to Figure 1B, which presents the chromatograms and peak capacities obtained with a glycoprotein BEH amide column containing the 300Å, wide-pore stationary phase. While the effective peak capacities for the IgG N-glycans are comparable, a marked improvement in peak capacity of approximately 17% is apparent in the separations of the Factor IX N-glycans when the wide-pore glycoprotein BEH amide 300Å, 1.7 μm column is used. The resolving power of the wide-pore, BEH amide column for the large N-glycans is noteworthy, in that it facilitates resolving several low abundance species. Figure 2 highlights some of the impacted regions of the chromatogram where there are improvements in the resolution of FA4G4S2, FA4G4S3, and FA4G4Lac1S3 N-glycans. In summary, when smaller, biantennary N-glycans are to be separated, a glycan BEH amide, 130Å, 1.7 μm stationary phase is an ideal choice due to its high surface area and high retentivity. For the characterization of large, tri- and tetra-antennary N-glycans, it is, however, advantageous to use the wide-pore amide stationary phase. Moreover, the wide-pore amide column is intended specifically for large biomolecule separations: ACQUITY UPLC Glycoprotein BEH Amide, 300Å, 1.7 μm stationary phase is ensured to have consistent batch-to-batch performance through stringent quality control testing involving a separation of ribonuclease B (RNase B) glycoforms at the intact protein level (see reference 8 for an example of this chromatography).8
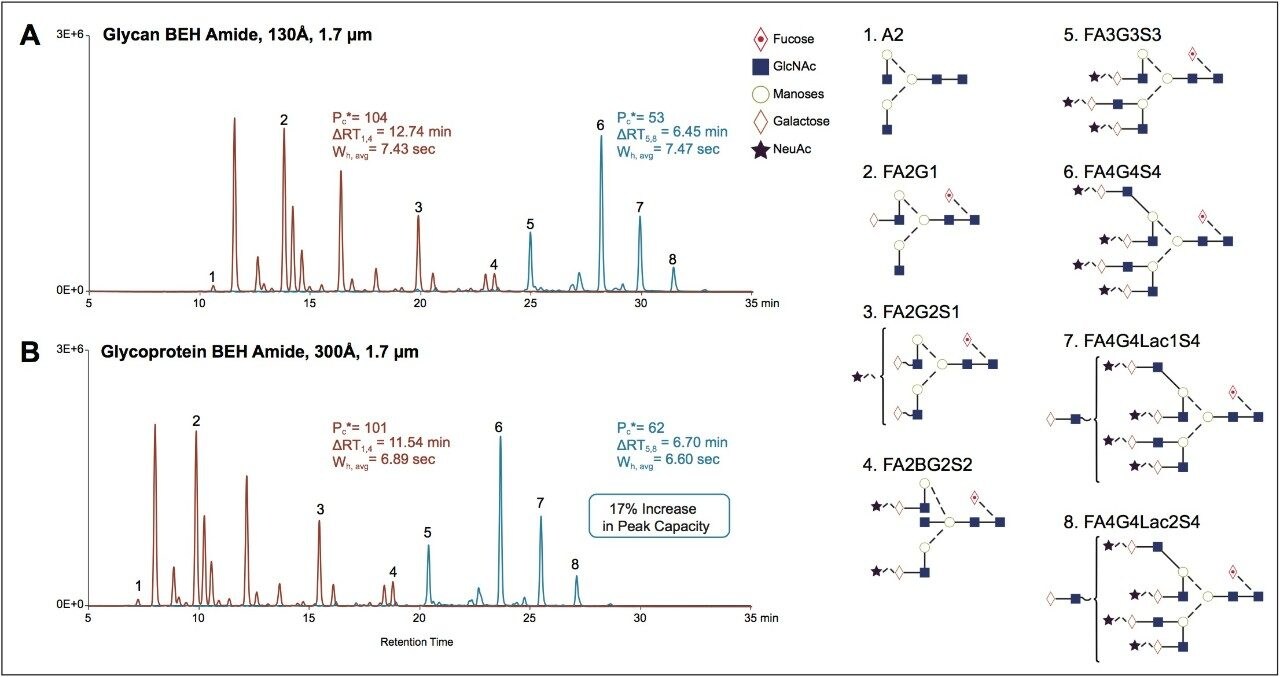
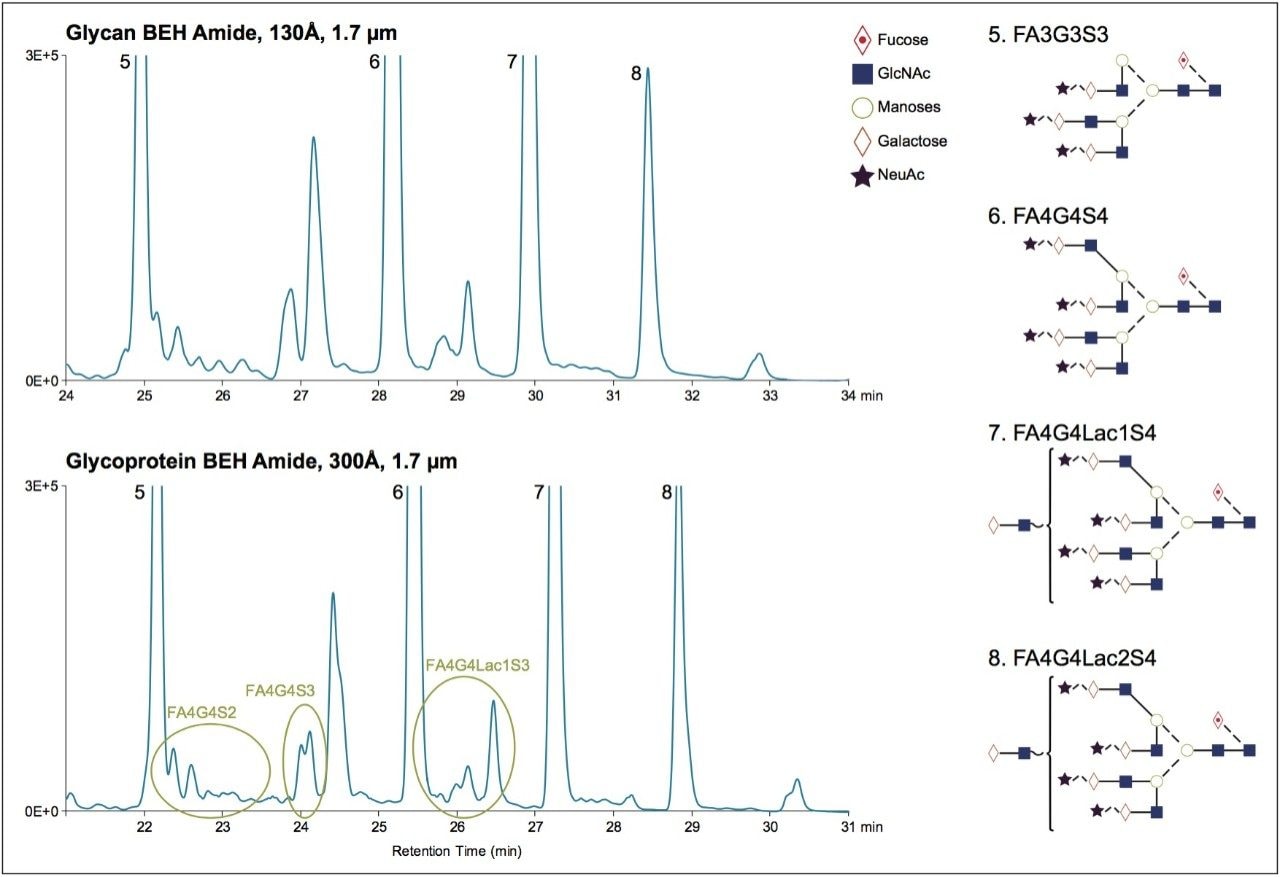
High molecular weight, tri- and tetra-antennary N-glycans are highly branched structures that adopt relatively large radii of hydration in solution. To achieve optimal HILIC separations of these large structures, we propose a column with a wide-pore amide bonded stationary phase, a glycoprotein BEH amide, 300Å, 1.7 μm column. For large glycan species, this column provides increases in peak capacity over a conventional pore diameter column of approximately 17%. Improved resolving power is particularly useful in this separation space as it is typified by highly complex glycan profiles. Most notably, these improvements in resolution should be of significant utility in the characterization and routine monitoring of biopharmaceuticals that are expressed with large, highly complicated N-glycan structures, such as coagulation Factor IX, erythropoietin, and darbepoetin.
720005381, April 2015