Fully Automated Bioanalytical Solid Phase Extraction Sample Preparation, using Extraction+ Connected Device with the Andrew+™ Pipetting Robot
Abstract
The following work demonstrates the capabilities of the Andrew+ Pipetting Robot in combination with the Extraction+ Connected Device for fully automated bioanalytical solid phase extraction (SPE) for a variety of pharmaceutical drugs from plasma using cartridge and 96-well SPE plate formats with subsequent LC-MS detection and analysis.
Benefits
- Automated standard curve and QC sample generation and solid phase extraction using Andrew+ Pipetting Robot and the Extraction+ Connected Device
- Easy-to-use OneLab™ Software with data visualization for creating and transferring methods
- Automation compatibility with SPE plates and cartridge formats
- Fully programmable vacuum pressure profiles with Extraction+ connected device reduce extraction performance variability
- Automated liquid handling and sample preparation increases efficiency, allowing the user to perform other tasks
- Full “walk-away” automation with no user intervention steps mitigates the risk of manual errors
Introduction
Sample preparation, which isolates target analytes from the matrix and removes as many interferences as possible, is a critical component of the bioanalytical workflow (Figure 1). To achieve accurate, precise, and reproducible results, a skilled and experienced analyst is often required, as any errors or inconsistencies in the execution of the sample preparation protocol need to be minimized to avoid their propagation through the entire process. This can result in inaccurate quantification and potential loss of time and resources to re-extract out-of-control batches. These errors or inconsistencies could include pipetting errors when generating calibration curves and quality control (QC) samples, missed or incorrectly labelled samples, incorrect addition of reagents, analyst technique-dependent inconsistencies, and other variables that can compromise results.
Introducing automation to the bioanalytical lab helps to minimize or eliminate some of these challenges, in addition to freeing up bioanalytical scientists for other tasks. Despite advances in automation of other parts of the bioanalytical workflow (e.g., instrumental analysis, autosamplers, data and result management, etc.), sample preparation is often still done manually, due to the time and automation expertise often required to develop robust automation protocols.
In this application, the Andrew+ Pipetting Robot was used to generate calibration curves and QC samples using a diversity of small molecule pharmaceutical drugs in plasma. The Andrew+ Pipetting Robot (Andrew+) and the new Extraction+ connected device (Extraction+) were then used to perform solid phase extraction (SPE) of these prepared samples. Extraction+ is the new, fully automatable SPE system solution used in combination with Andrew+. Extraction+ comprises a vacuum manifold with a “collar lifter” and a “parking spot” (idle position), a controllable vacuum pump, and a waste collector within the manifold base that is linked to an external waste container (GL45-threaded bottle). It provides full “walk-away” SPE sample preparation in both cartridge and 96-well plate formats. Using this SPE system solution resulted in an accurate and precise generation of calibration curves, QC samples, and extraction of analytes from plasma. The resulting calibration curves showed excellent precision (RSDs ±15%) and linearity (>0.99), while quality control accuracy ranged from 95.8–107.5%, with RSDs ≤5.0% for the low, mid, and high QC. These results are well within FDA recommendations for small molecule LC-MS bioanalytical assay performance.

Experimental
Materials
All target pharmaceuticals were purchased from Sigma Aldrich. Stock solutions (1 mg/mL) of each analyte were prepared in methanol. A 10 mL stock solution of all analytes (100 μg/mL) was made in methanol. Rat plasma (K3EDTA) was purchased from Innovative Research. Daily working solutions for curve and QC generation were prepared in plasma. For the cartridge protocols, calibrators were prepared in duplicate ranging from 5–200 ng/mL. QC plasma samples were prepared in triplicate at 10 (Low QC), 50 (mid QC), and 150 ng/mL (high QC). For the 96-well SPE plate protocols, calibrators were prepared in duplicate ranging from 5–500 ng/mL. QC plasma samples were prepared in triplicate at 20 (Low QC), 50 (mid QC), and 400 ng/mL (High QC). LC-MS grade formic acid and phosphoric acid were purchased from Sigma Aldrich.
Automation Platform
The Andrew+ Pipetting Robot, equipped with the new Extraction+ connected device and controlled with the cloud-based OneLab Software, was used to design and execute the sample preparation and SPE extraction protocols.
SPE Extraction
Extraction protocols
The same reversed-phase (RP) SPE extraction method was performed using Oasis HLB 30 mg plates (p/n: WAT058951) or Oasis HLB 1 cc cartridges (p/n: 186001879). The loading step used a 1.0 mL volume of 1:1 plasma diluted with 4% phosphoric acid aqueous solution (0.5 mL undiluted plasma). Samples were then washed with 1 mL of 95:5 Water:MeOH, eluted with 2 x 250 µL aliquots of 100% methanol and diluted with 500 µL water.
The protocols and visual representation of the four OneLab protocols used for these bioanalytical plate and cartridges SPE sample preparation are shown in Figures 2–9. For each format, a single protocol was used for sample preparation and dilution followed by a separate protocol for SPE extraction performed with Andrew+ configured with Extraction+.
Protocol 1 - Curve and QC Preparation for HLB 30 mg Plate Extraction – Protocol Visualization
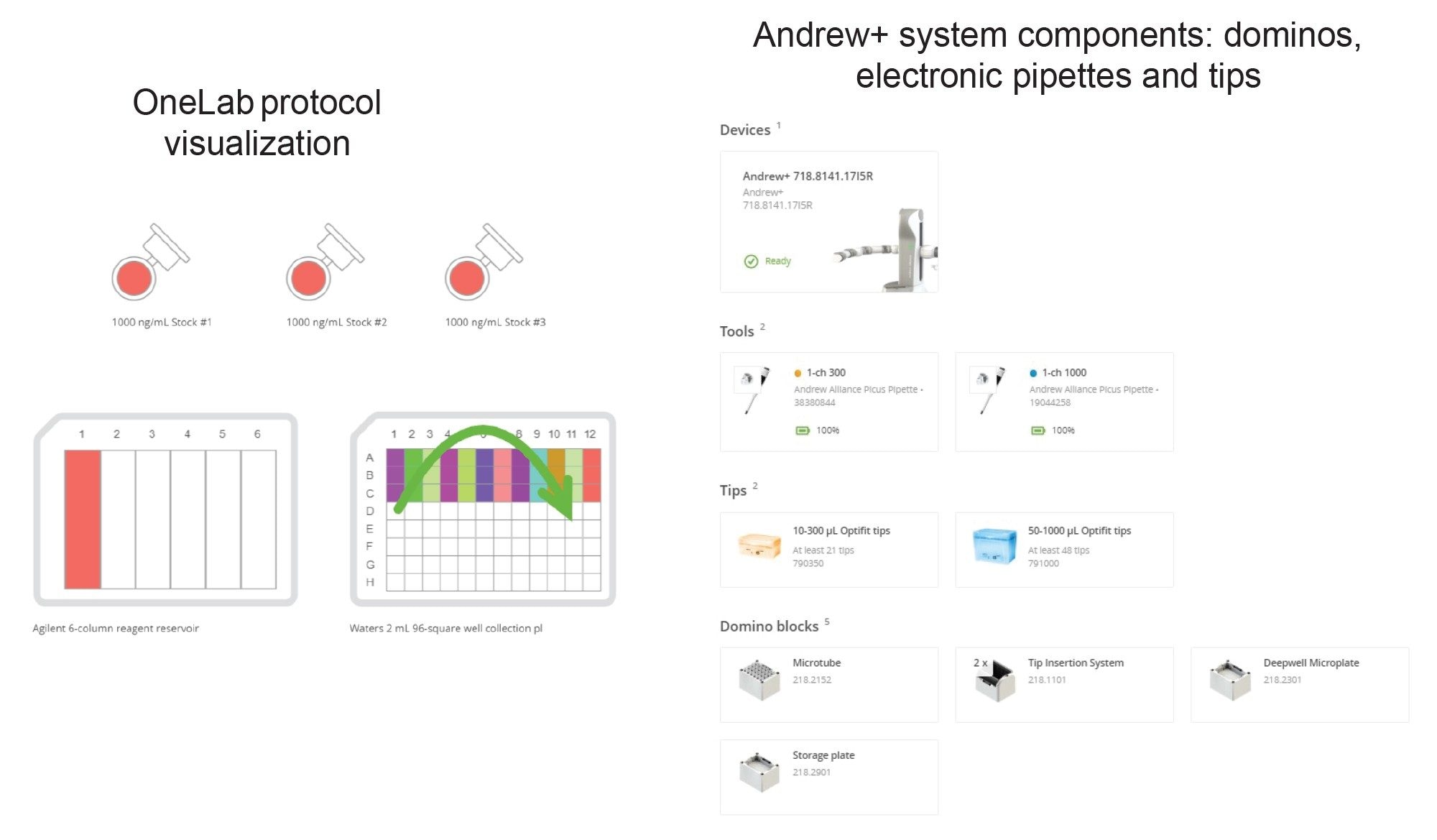
Protocol 1 - Curve and QC Preparation for HLB 30 mg Plate Extraction – Andrew+ Deck Layout

Position | Component Layout
1–2 Tip Insertion System Dominos
3 Storage Plate Domino
4 Microtube Domino
5 Deepwell Microplate Domino
Figure 3. The Andrew+ deck layout for sample dilutions is shown above, illustrating the placement of all items. Dominos required are listed below the figure.
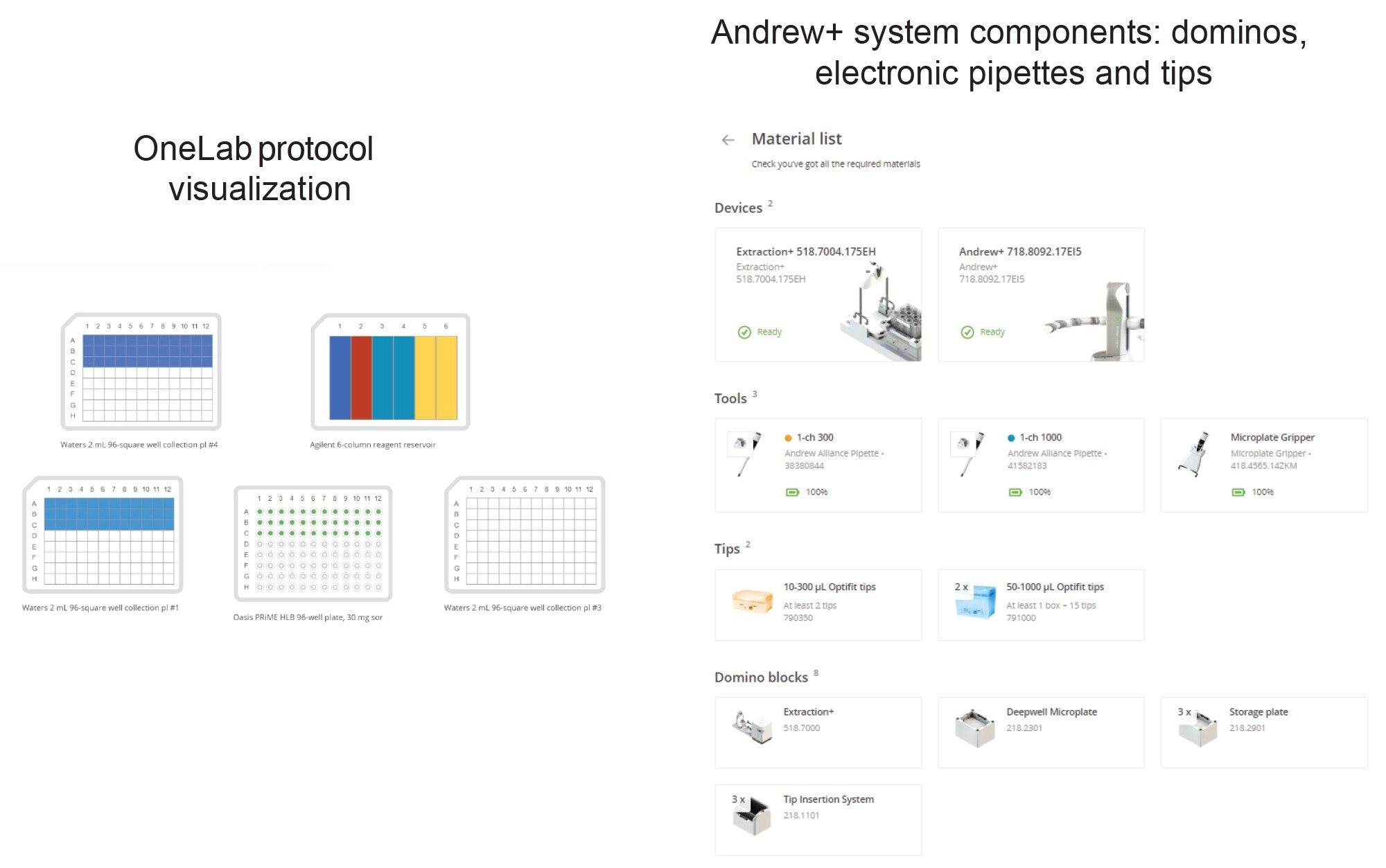
Protocol 2 – Oasis HLB 30 mg Plate Extraction – Protocol Visualization
Other Consumables
Waters Oasis HLB 96-well plate 30 mg sorbent/well | p/n: WAT058951
Waters 2 mL square collection plate | p/n: 1860002482
Agilent 6-Column Reagent Reservoir | p/n: 201-284-100
Figure 4. The OneLab method’s equipment list and protocol visualization for plate extraction (SPE protocol) are shown above. Pipettes, pipette tips, Dominos, and labware required are shown on the right side.
Protocol 2 – Oasis HLB 30 mg Plate Extraction – Andrew+ Deck Layout
Position | Component Layout
1–3 Tip insertion System Dominos
4 Deepwell Microplate Domino
5 Extraction+ connected device
6-7 Storage Plate Dominos
8 Deepwell Microplate Domino
Figure 5. The OneLab Protocol’s deck layout for plate extraction (SPE protocol) is shown above, illustrating the placement of all items. Dominos and device required are listed below the figure.
Protocol 3 - Curve and QC Preparation | 1 cc Cartridge Extraction – Protocol visualization
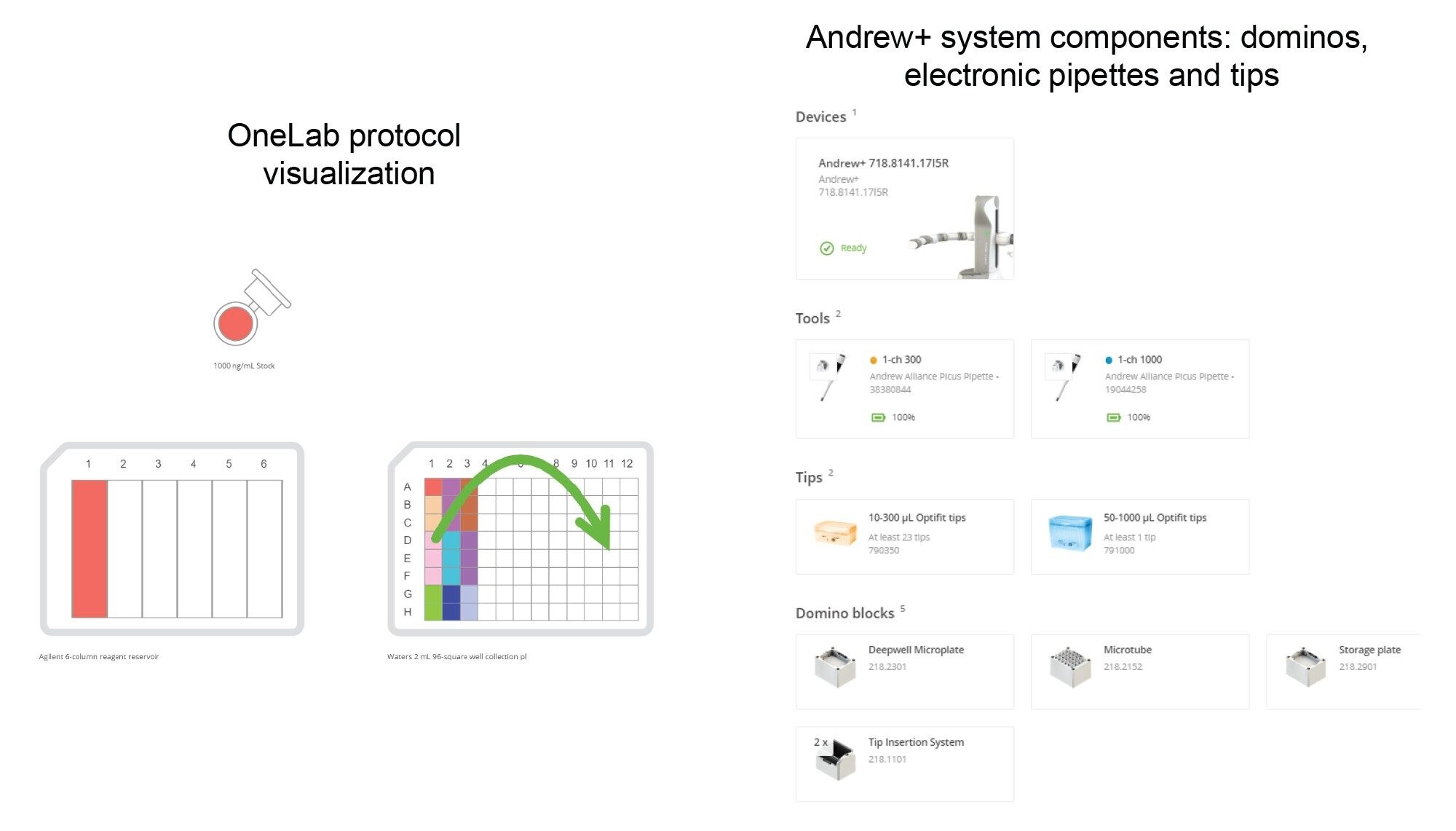
Other Consumables
Eppendorf 2 mL Safe-Lok Tube | p/n: 0030120094
Waters 2 mL square collection plate | p/n: 1860002482
Agilent 6-Column Reagent Reservoir | p/n: 201-284-100
Figure 6. The OneLab method’s equipment list and protocol visualization for sample dilutions are shown above. Pipettes, pipette tips, dominos, and labware required are shown on the right side.
Protocol 3 - Curve and QC Preparation | Oasis HLB 1 cc Cartridge Extraction | Andrew+ Deck Layout

Position | Component Layout
1–2 Tip Insertion System Domino
3 Storage Plate Domino
4 Microtube Domino
5 Deepwell Microplate Domino
Figure 7. The OneLab Protocol’s deck layout for sample dilutions is shown above, illustrating the placement of all items. Dominos required are listed below the figure.
Protocol 4 – Oasis HLB 1 cc Vac Cartridge extraction – Protocol Visualization
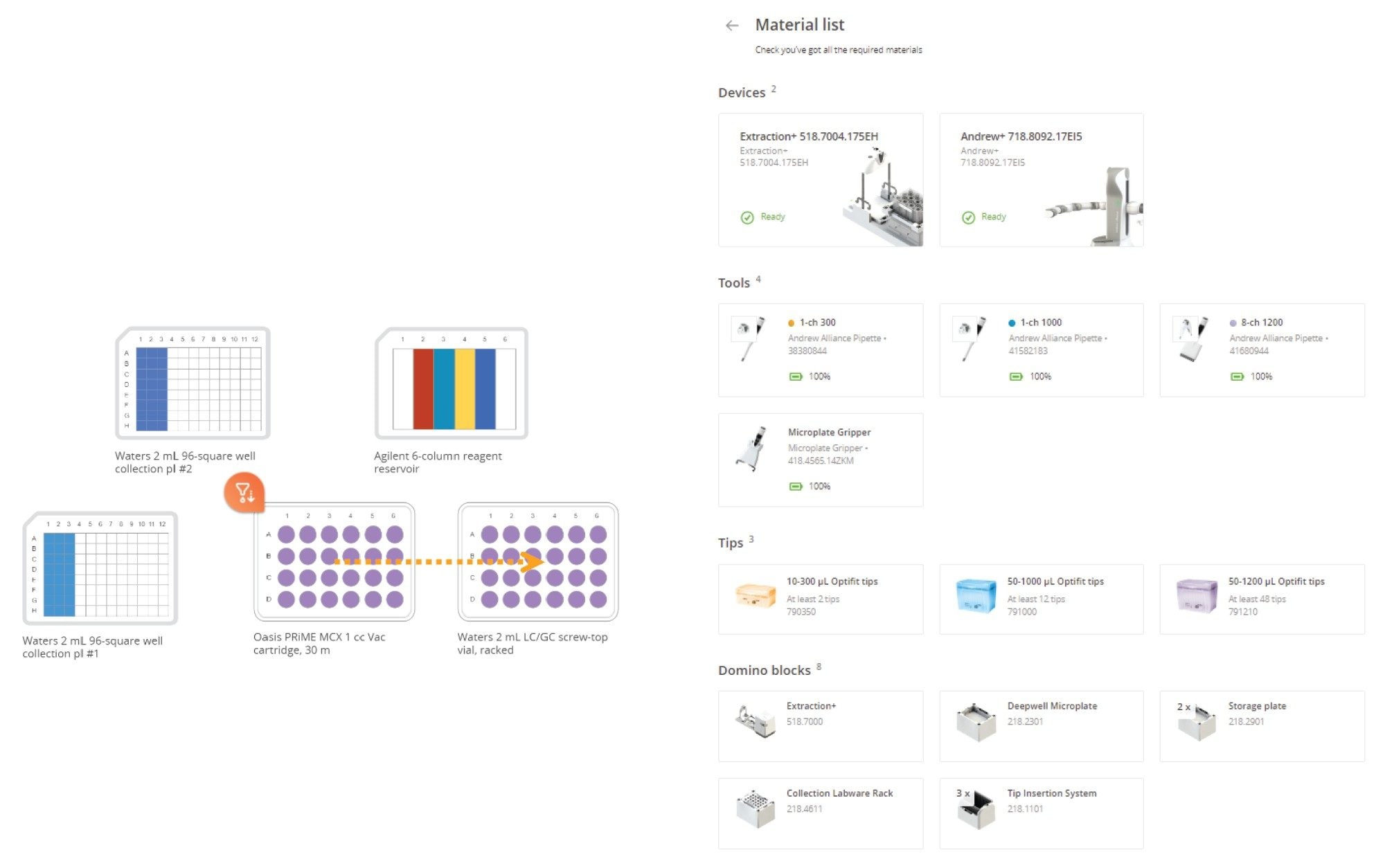
Other Consumables
Waters Oasis HLB 1 cc Vac cartridge 30 mg sorbent | p/n: WAT094225
Waters 2 mL square collection plate | p/n: 1860002482
Agilent 6-Column Reagent Reservoir | p/n: 201-284-100
TruView LCMS Certified clear glass 12 x 32 mm Max Recovery Vial | p/n: 186005662CV
Figure 8. The OneLab method’s equipment list and protocol visualization for cartridge extraction (SPE protocol) are shown above. Pipettes, pipette tips, dominos, and labware required are shown on the right side.
Protocol 4 – Oasis HLB 1 cc Vac Cartridge Extraction Using Extraction+ Connected Device – Andrew+ Deck Layout
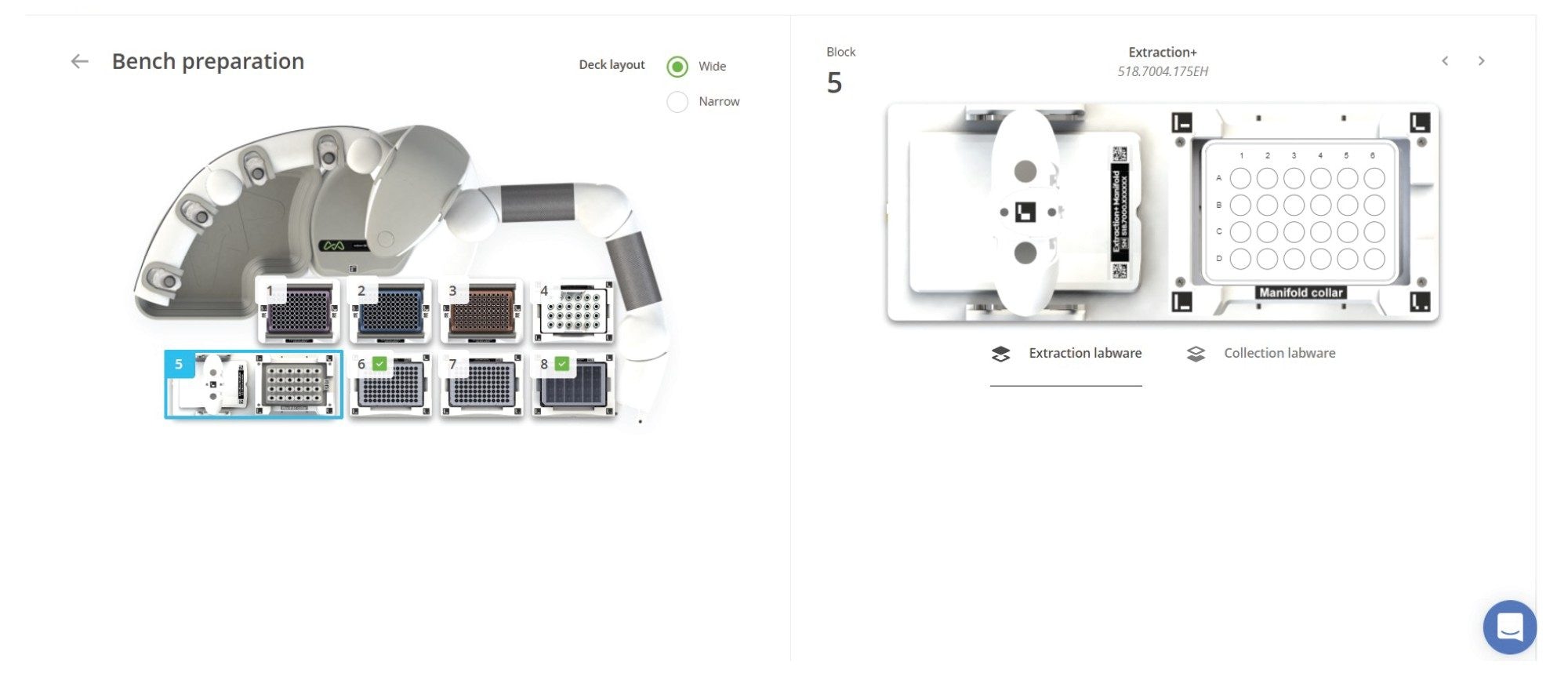
Position | Component Layout
1–3 Tip Insertion System Dominos
4 Collection Labware Rack Domino
5 Extraction+ connected device
6–7 Storage Plate Dominos
8 Deepwell Microplate Domino
Figure 9. The OneLab Protocol’s deck layout for the cartridge extraction (SPE protocol) is shown above, illustrating the placement of all items. Dominos and device required are listed below the figure.
Chromatography & MS/MS Conditions
|
LC Conditions |
|
|
LC system: |
ACQUITY UPLC I-Class |
|
Mobile phase A: |
0.1% Formic acid in 100% MilliQ water |
|
Mobile phase B: |
0.1% Formic acid in 100% Acetonitrile |
|
Weak wash solvent: |
Water:methanol (90:10 v/v) |
|
Strong wash solvent: |
Acetonitrile:isopropanol:water:methanol (25:25:25:25 v/v/v/v) |
|
Detection: |
Xevo TQ-XS Mass Spectrometer |
|
Column(s): |
ACQUITY UPLC HSS PFP Column, 1.8 µm, 2.1 mm x 50 mm (p/n: 186005965) |
|
Column temp.: |
35 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
5 µL |
|
Flow rate: |
0.5 mL/min |
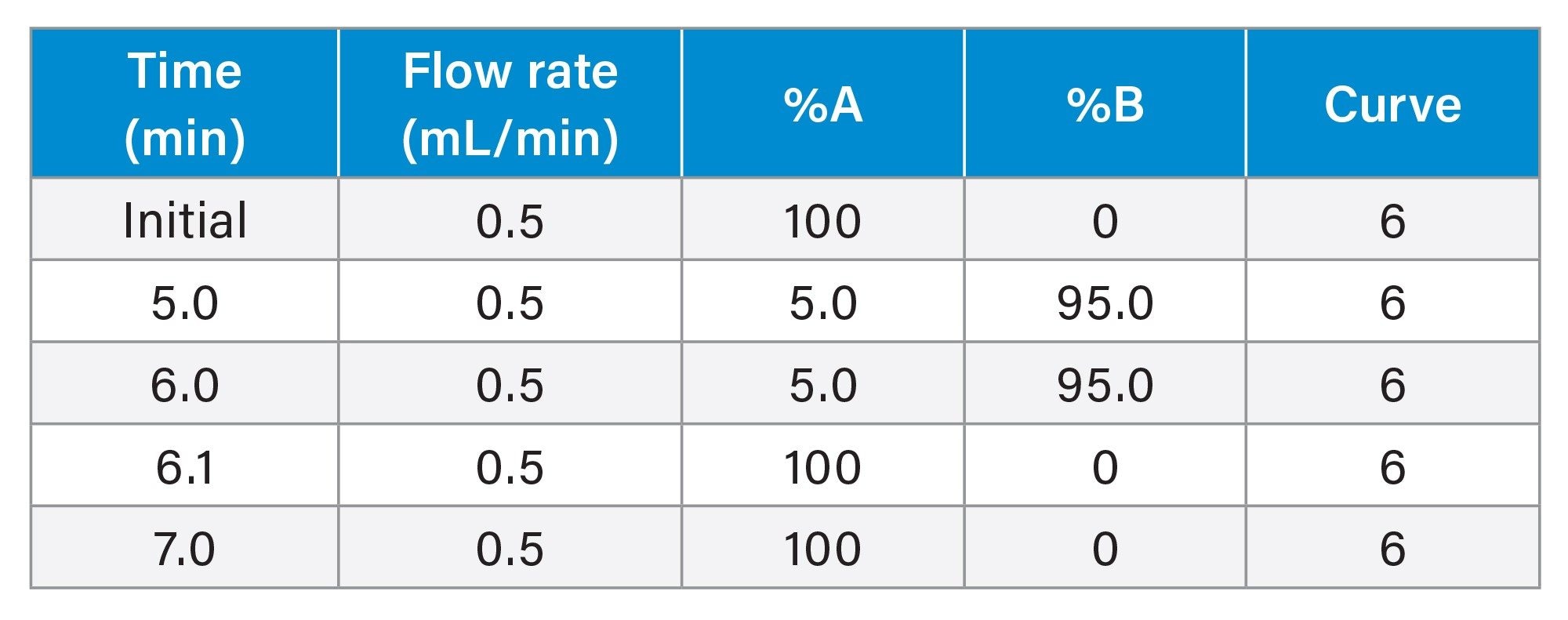
LC Gradient
MS Conditions
|
MS system: |
Xevo™ TQ-XS |
|
Ionization mode: |
ESI+ |
|
Acquisition range: |
MRM |
|
Capillary voltage: |
2.0 kV |
|
Cone voltage: |
60 V |
|
Desolvation temp: |
500 °C |
|
Desolvation flow: |
1100 L/Hr |
|
Cone gas flow: |
150 L/Hr |
|
Collision gas flow: |
0.2 mL/min |
|
Nebulizer gas flow: |
7 Bar |
Data Management
|
Instrument control software: |
MassLynx™ (v4.2) |
|
Quantification software: |
TargetLynx™ |
LC-MS Analysis
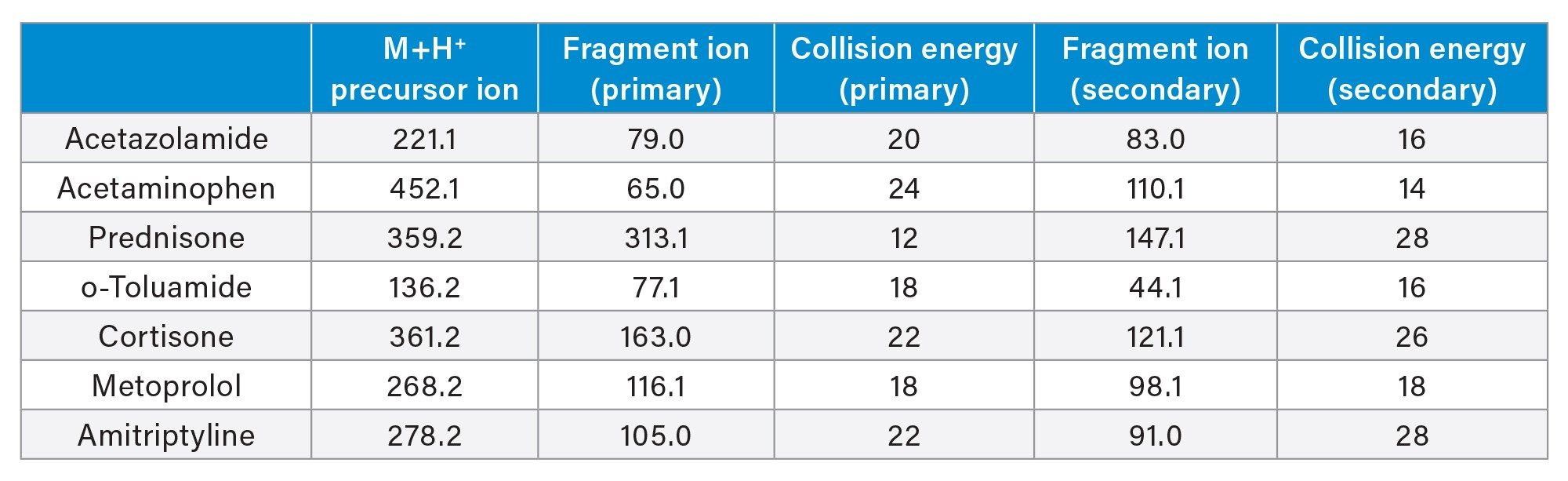
The chromatographic separation was performed using a Waters ACQUITY UPLC I-Class and ACQUITY UPLC HSS PFP Column (1.8 µm, 2.1 x 50 mm,) with gradient elution using water and acetonitrile mobile phases containing 0.1% formic acid. The flow rate was set at 0.5 mL/min with the column temperature set at 35 °C. Detection of pharmaceutical analytes was performed with a Waters Xevo TQ-XS Mass Spectrometer (ESI+) using Multi Reaction Monitoring (MRM) of the individual analytes. Two MRM transitions per analyte were chosen for MS detection (quantification and confirmation). The MS conditions for each pharmaceutical are listed in Table 2.
Results and Discussion
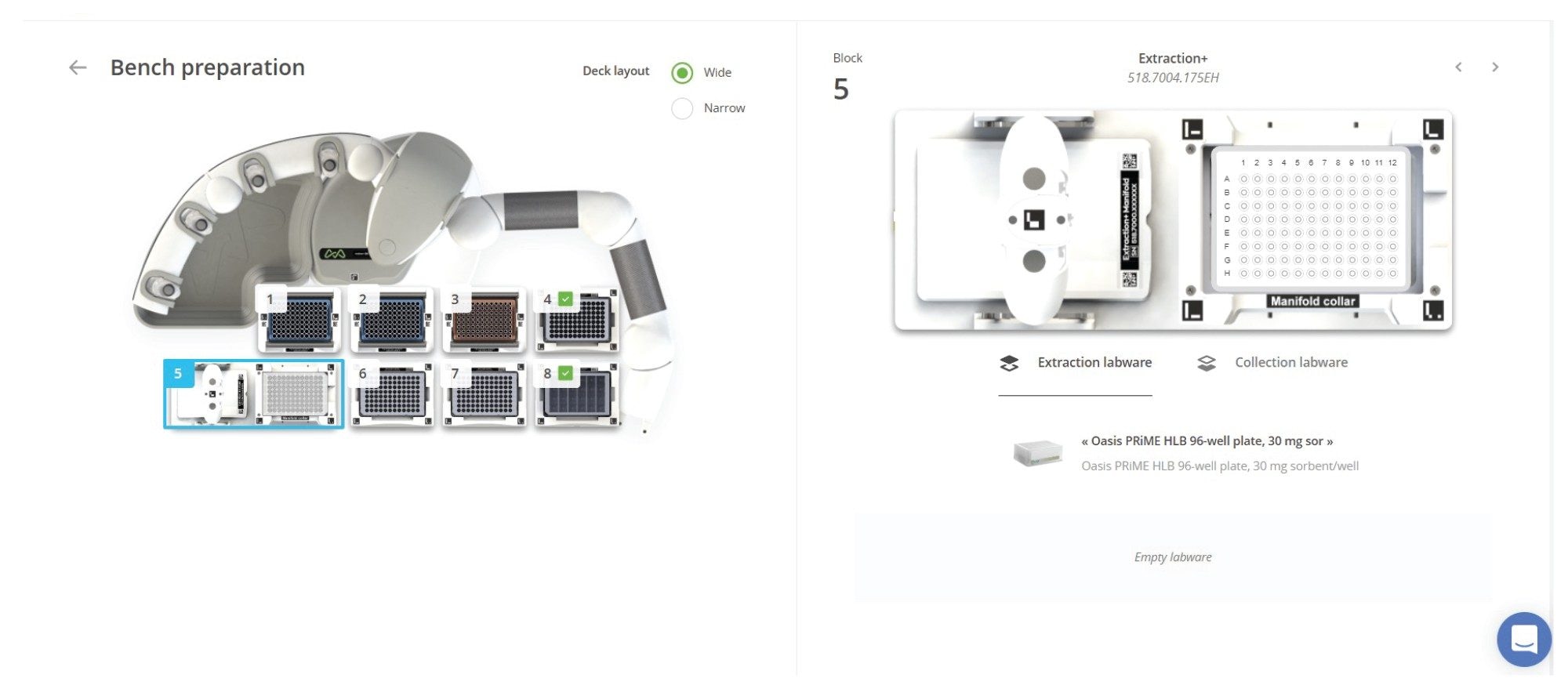
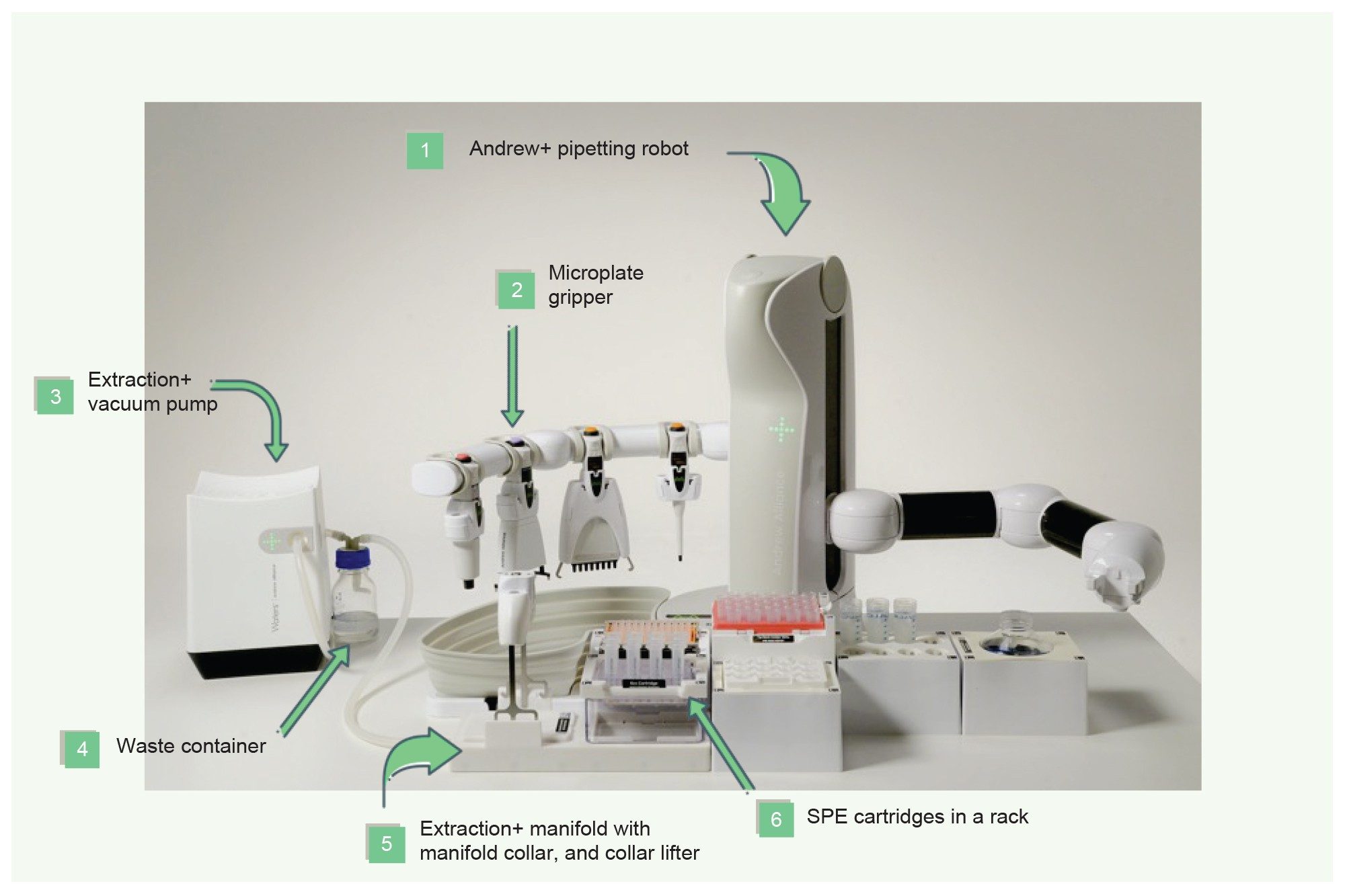
Extraction+ is the new fully automatable SPE system solution combined with Andrew+, which eliminates the need for user intervention during SPE sample preparation and extraction (Figure 10). The Extraction+ connected device is comprised of two modules: A carefully designed SPE manifold and vacuum pump, both controlled via the OneLab Software. When used with the Andrew+ pipetting robot, one can carry out the liquid handling and the sample extraction, providing complete walk-away sample preparation. Key features of the Andrew+ Pipetting robot combined with the Extraction+ connected device include compatibility with both plates and cartridges, flow through waste collection, full vacuum control of the protocols, and, most importantly, full walk-away automation. The flow-through waste collection eliminates the need to place and remove waste collection labware. The integrated collar lifter moves the manifold collar to and from the manifold base and the Andrew+ microplate gripper is used to place collection plates or racked HPLC vials in the manifold (active position). This combination eliminates the need for any user intervention during the extraction process.
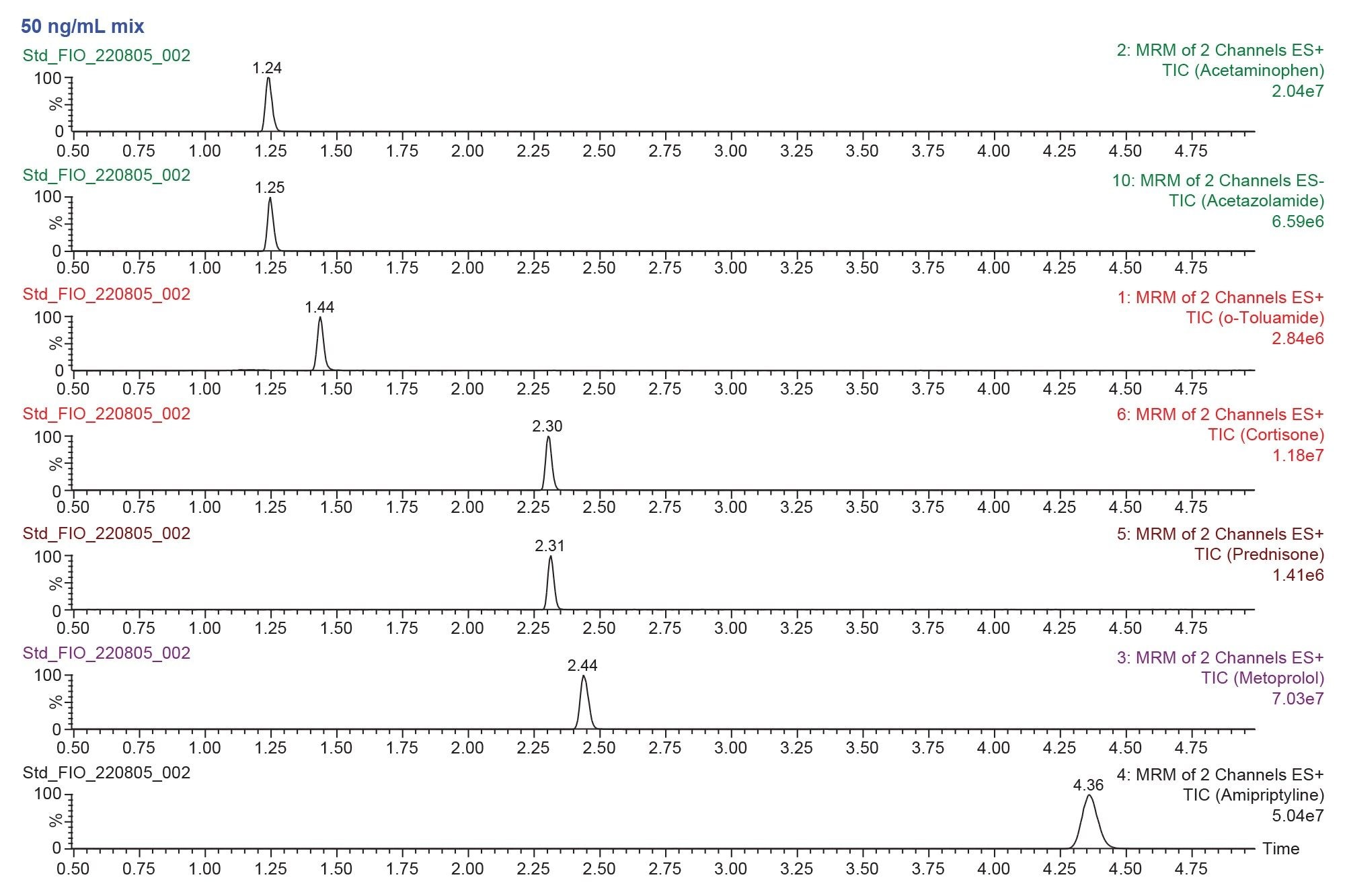
Chromatography
Figure 11 shows the chromatography of the compounds used for this application. They had a diverse range of polarities, as can be seen by their retention time differences. They also included a mix of charged and neutral compounds.
Sample preparation and SPE Cartridge/Plate Extraction
The Andrew+ and the OneLab Software were able to successfully prepare curve and QC dilutions and extract those prepared plasma samples in a fully automated manner using the new Extraction+ capabilities. Automatic mixing of dilutions ensured consistent performance and is seen in the accuracy of the quantitative results. Some of the additional benefits include the absolute certainty of dilutions and sample integrity. Once programmed, there is no worry about adding the wrong amount of sample or the wrong reagent. Likewise, there is no risk of mixing up or transposing samples or incorrectly spiking all or some of a batch. Finally, the vacuum extraction steps are optimized for the assay and consistent sample to sample and batch to batch, again ensuring reliable performance.
Quantitative Results
Table 2 below shows a summary of the quantitative results from the Andrew+ preparation of standards and QC samples and subsequent extraction of plasma samples using Waters HLB 96-well plate with the Extraction+. Accuracy values ranged from 92.1–108.3%. The QC results were precise as well, with all %RSDs except for one under 5%. These results meet the recommended limits for small molecule bioanalytical method validation. The high degree of precision in the analytical results is a testament to the consistency of the Andrew+ Pipetting Robot configured with the Extraction+ device for sample preparation and SPE automation. Calibration curves for selected compounds are shown in Figure 12.
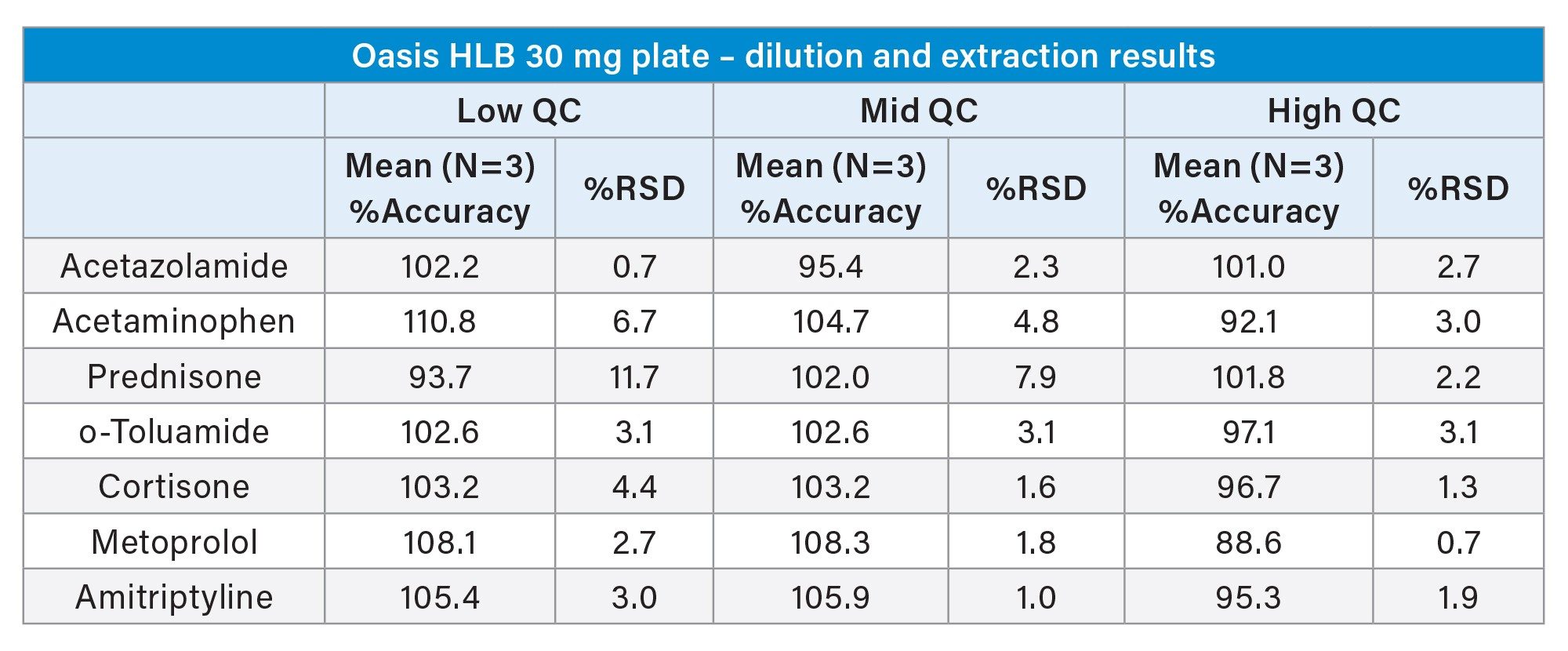
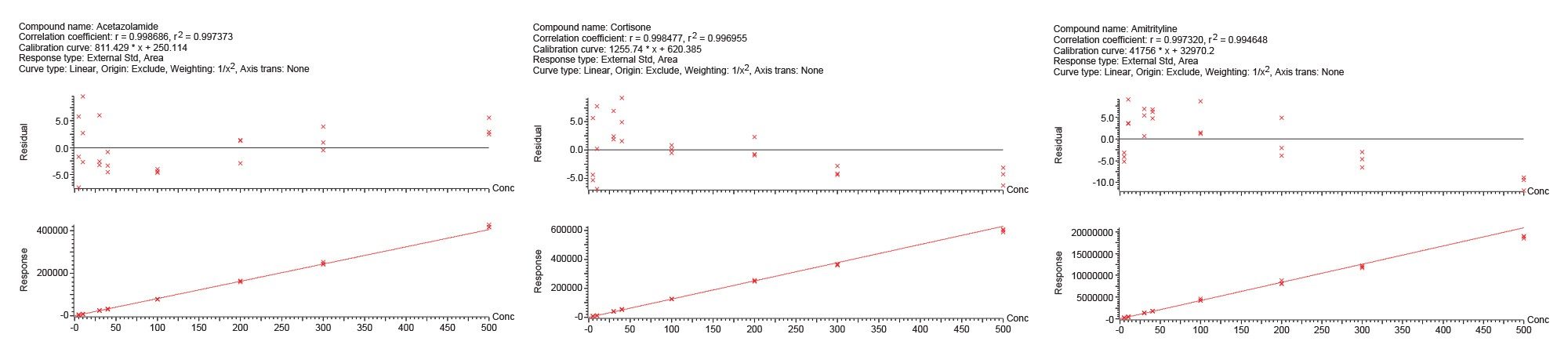
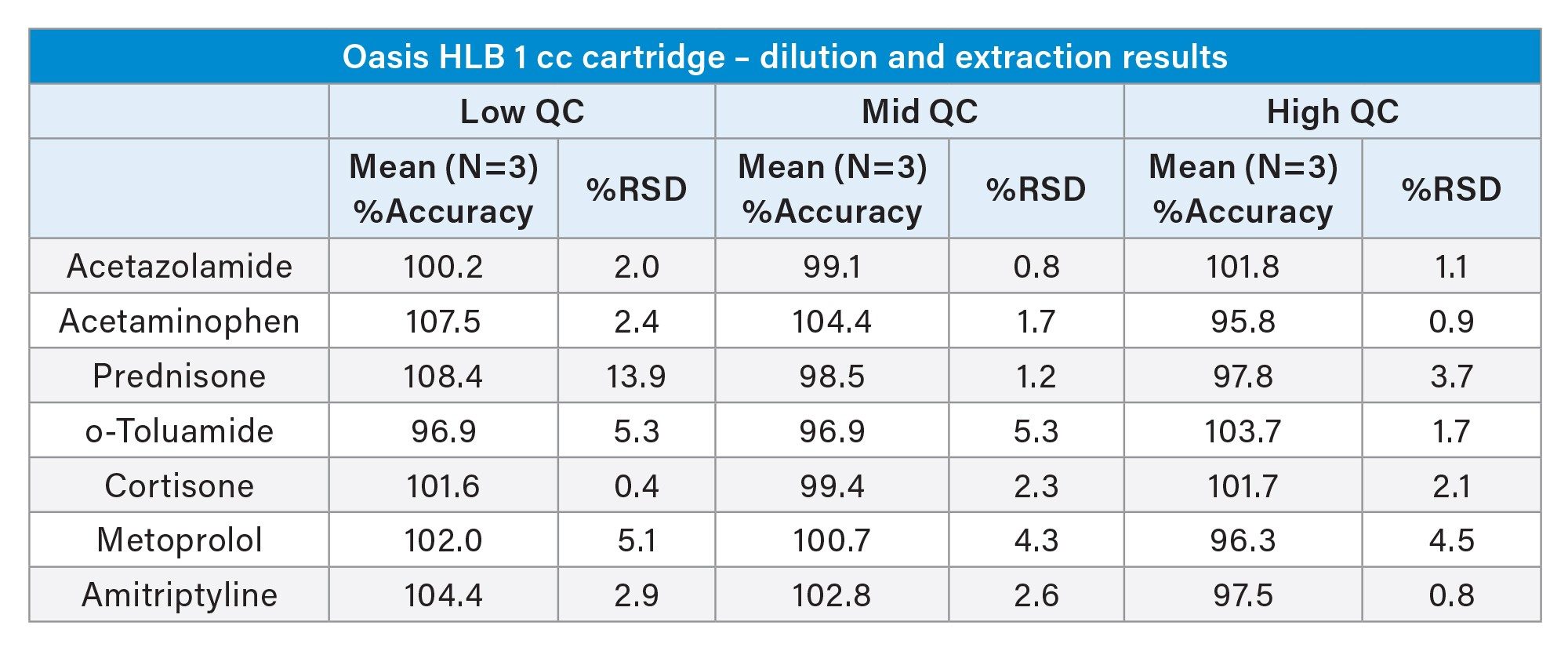
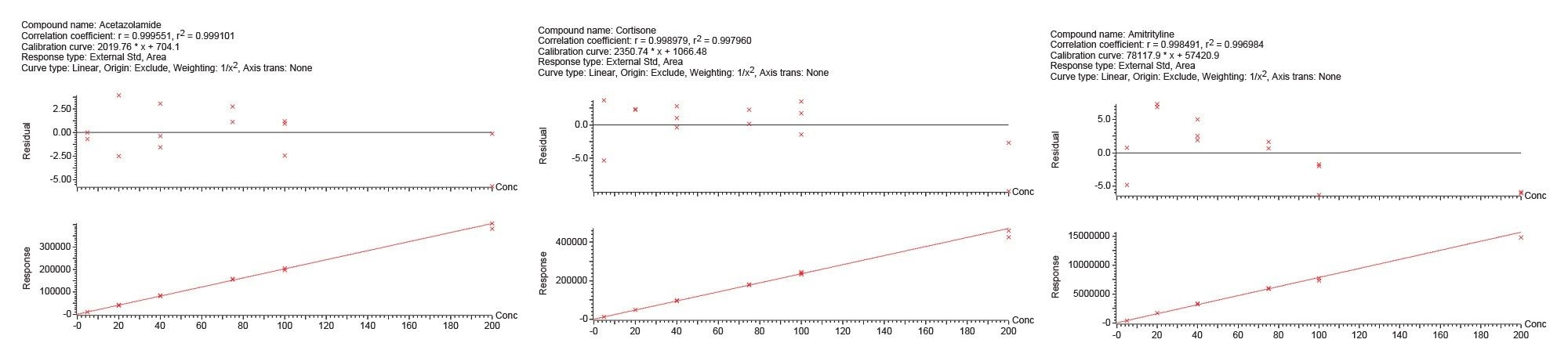
Table 3 below shows a summary of the quantitative results from the Andrew+ preparation of standards and QC samples and subsequent extraction of plasma samples using Waters HLB 1 cc cartridges and the Extraction+ connected device. Accuracy values ranged from 88.6–110.8%. The results were precise as well, with all %RSDs except for one ≤5%. These results again meet the recommended guidelines for small molecule bioanalytical method validation. Calibration curve results for selected compounds are shown in Figure 13.
Conclusion
This application highlights the successful use of the Andrew+ Pipetting Robot configured with the the Extraction+ connected device for fully automated sample preparation and SPE extraction for both cartridges and 96-well plate formats. The accurate and precise data shown in Tables 2 and 3 demonstrate not only the accuracy and precision of the pipetting steps, but also the performance of the extraction protocols performed with Extraction+. The closeness of the results demonstrates excellent performance agnostic of format (cartridge vs. plate). A key feature of the Extraction+ is the ability to perform fully automated SPE extractions using plate or cartridge, with no required user intervention. This enables scientists in the laboratory to focus on other areas rather than repetitive manual tasks.
The features highlighted and the data shown in this application demonstrate that Andrew+ and the Extraction+ SPE system are capable of fully automated standard curve and QC sample preparation and SPE regardless of extraction labware format. These features, combined with the intuitive OneLab cloud-based Software enable easy adoption of laboratory automation, maximizing productivity, reducing errors, and ensuring an overall analytical performance of bioanalytical LC-MS workflows.
720007712, September 2022