
Numerous laboratory and field studies have been performed to investigate the transport of glyphosate and/or AMPA to the aquatic environment indicating some recognition and concern that these substances can move towards surface waters.
The difficulties associated with determination of these compounds at trace levels in water samples are related to their high solubility in water, ionic nature, and chelation with metal ions. All three compounds can be derivatized to less polar compounds for improved retention and separation using solid phase extraction (SPE) and reversed-phase liquid chromatography (LC). Fluorenylmethyloxycarbonyl (FMOC) chloride is the most common pre-column derivatization reagent used for this analysis, and it can be successfully used in combination with LC-MS/MS for the determination of all three compounds in one method as part of water monitoring programs.
This application note describes a method for the determination of glyphosate, AMPA, and glufosinate in water samples, without SPE, after derivatization with FMOC, by LC-MS/MS using the ACQUITY UPLC I-Class System coupled to the Xevo TQ-XS using a novel ionization technology, UniSpray.
The goal of any extraction technique is to achieve efficient and reproducible recovery for all relevant analytes. As in previous work, the wash protocol was modified from the traditional MCX technique to accommodate the benzodiazepines.2 Figure 4 shows the mean extraction recoveries of the entire panel of compounds from six different lots of urine. With the exception of meprobamate and norpropoxyphene, all compounds but two (MDMA and EDDP) had recoveries greater that 70%. Extraction efficiencies were also consistent. Coefficients of variation (%CV) were less than 10% for all quantitative compounds. Recovery data for individual batches followed the same pattern. These highly efficient recoveries across different matrix lots demonstrate the robustness of the extraction technique and are important for quantification of these compounds in samples from different sources.
The widespread use of pesticides for agricultural and nonagricultural purposes has resulted in the presence of their residues in surface and ground water resources. Glyphosate is one of the most widely used broad-spectrum herbicides around the globe. Aminomethyl-phosphonic acid, commonly known as AMPA, is the major metabolite of glyphosate in the environment. Glufosinate-ammonium is another highly effective herbicide used to control weeds in many countries around the world and it has a similar chemical structure. The diverse and intensive use of such herbicides implies that residues have the potential to reach surface waters throughout the year from indirect routes of entry such as spray drift, runoff and drainage, as well as point source contamination. Numerous laboratory and field studies have been performed to investigate the transport of glyphosate and/or AMPA to the aquatic environment indicating some recognition and concern that these substances can move towards surface waters. At the same time, glyphosate and AMPA are only sporadically detected in deep groundwater systems and at low concentrations indicating that the leaching of these compounds is generally unlikely and probably negligible.1
The difficulties associated with determination of these compounds at trace levels in water samples are related to their high solubility in water, ionic nature, and chelation with metal ions. All three compounds can be derivatized to less polar compounds for improved retention and separation using solid phase extraction (SPE) and reversed-phase liquid-chromatography (LC). Fluorenylmethyloxycarbonyl (FMOC) chloride is the most common pre-column derivatization reagent used for this analysis, and it can be successfully used in combination with LC-MS/MS2,3,4 for determination of all three compounds in one method as part of water monitoring programs.
Public water suppliers abstract raw water from a range of different sources depending on local availability. In some countries, supplies are taken almost entirely from groundwater, while in other countries surface waters (rivers, canals, lakes, or reservoirs) are the predominant source of drinking water. The presence of pesticides in water is regulated through different directives. Member States have the obligation to ensure that regular monitoring of the quality of water is carried out in order to check that the water available to consumers meets the requirements of the Drinking Water Directive.5 This sets a maximum limit of 0.1 µg/L for individual pesticide residues present in a sample (0.5 µg/L for total pesticides). In general, the World Health Organization (WHO) guidelines for drinking water and the opinion of the Commission's Scientific Advisory Committee are used as the scientific basis for quality standards in drinking water. The Water Framework Directive (WFD),6 which aims to improve the quality of water across Europe, deals with surface waters, coastal waters, and groundwater, and seeks to provide a good chemical status of water across Europe. Member States must identify River Basin Specific Pollutants and set their own national environmental quality standards (EQSs) for these substances. Specific Pollutants are substances that may have a harmful effect on biological quality and which have be identified as being discharged to the water environment in significant quantities in the Member States. Values for these EQS vary across Europe; for example, the long term mean EQS for glyphosate in the UK is 196 µg/L7 but it is 28 µg/L in France and Germany.8 Hence, there is a need for reliable analytical methods for monitoring these polar herbicides in drinking, surface, and ground waters.
This application note describes a method for the determination of glyphosate, AMPA, and glufosinate in water samples, without SPE, after derivatization with FMOC, by LC-MS/MS on Waters ACQUITY UPLC I-Class System coupled to the Xevo TQ-XS using a novel ionization technology, UniSpray.
The issue of complexation of glyphosate with various cations resulting in low recoveries has been well established in environmental water analysis.3 All water samples (12 mL) were filtered (0.22 µm cellulose membrane filter); salts and metals removed using ion exchange (Dionex OnGuard II Na ion exchange syringe cartridge) and stored in polypropylene (PP) containers. A test portion from each filtered water sample was treated using the derivatization procedure shown in Figure 1. Solutions of standards were prepared in a sample of drinking water, internal standards were added and solutions derivatized using the same procedure.
The accuracy of the method was assessed by analyzing water samples spiked with the compounds of interest at various concentrations. Solutions of standards were prepared over the range 0.02 to 2.0 µg/L, in drinking water to determine the concentration of analytes in the recovery spikes and to evaluate linearity of response.

|
UPLC system: |
ACQUITY UPLC I-Class with FTN Sample Manager equipped with a 50 μL extension loop and 250 μL sample syringe |
|
Column: |
ACQUITY UPLC BEH Phenyl, 1.7 μm, 2.1 x 100 mm |
|
Mobile phase A: |
5 mM ammonium acetate (aq.), pH 9 (using 25–28% NH4OH solution) |
|
Mobile phase B: |
Methanol |
|
Flow rate: |
0.4 mL/min |
|
Injection volume: |
50 μL |
|
Column temp.: |
50 °C |
|
Sample temp.: |
10 °C |
|
Run time: |
16 min |
|
Time (min) |
%A |
%B |
Curve |
|---|---|---|---|
|
0 |
90 |
10 |
– |
|
5 |
54 |
46 |
6 |
|
7 |
54 |
46 |
6 |
|
8 |
0 |
100 |
6 |
|
9.5 |
0 |
100 |
6 |
|
11 |
90 |
10 |
1 |
|
MS system: |
Xevo TQ-XS |
|
Source: |
UniSpray |
|
Ionization mode: |
US |
|
Capillary voltage: |
3.0 kV |
|
Desolvation temp.: |
550 °C |
|
Desolvation gas flow: |
1000 L/Hr |
|
Source temp.: |
150 °C |
|
Cone gas flow: |
150 L/Hr |
|
Cone voltage: |
14 V |
|
Collision gas flow: |
0.14 mL/min |
|
Nebulizer gas pressure: |
7 Bar |
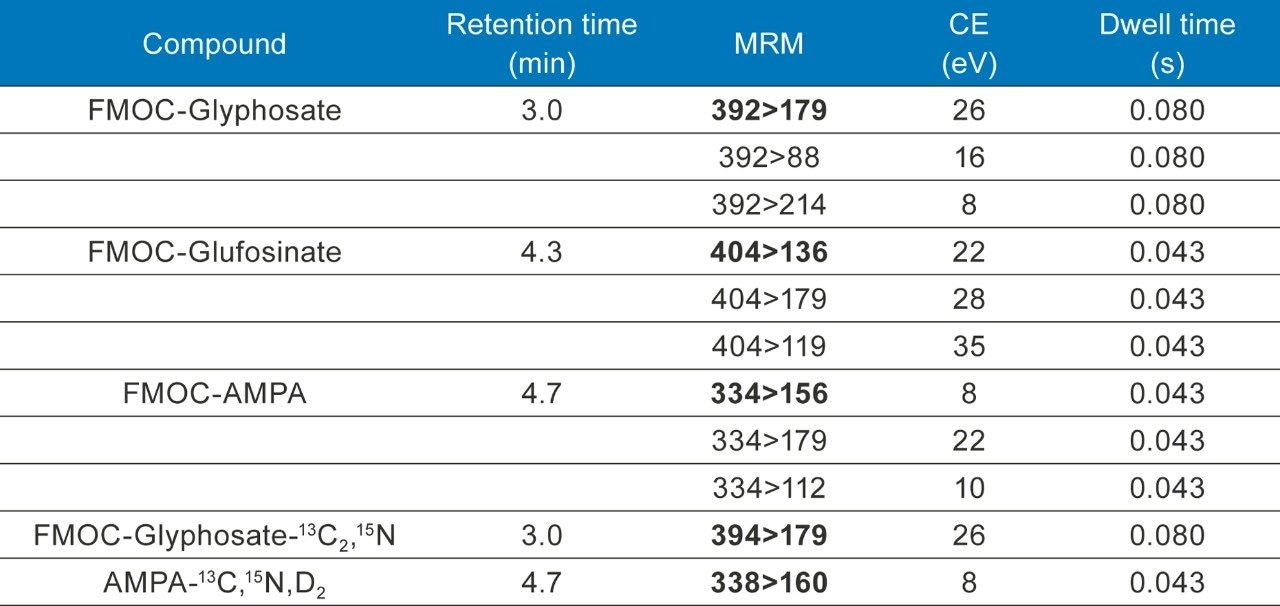
Data were acquired using MassLynx MS Software (v4.2) and processed using TargetLynx XS Application Manager. The selection of MRM transitions and the optimization of critical parameters was performed by infusion of individual solutions of all the analytes and evaluation of the data by IntelliStart Software to automatically create acquisition and processing methods. Table 1 summarizes conditions for all MRM transitions including the retention times. The optimum dwell time was set automatically using the Autodwell function. For this work, stable isotope labeled AMPA was used as an internal standard for the determination of glufosinate.*
*Glufosinate-D3 is now available for a number of suppliers.
UniSpray is a novel, proprietary, ionization source that provides increased ionization efficiency.9 The unique geometry of the UniSpray ion source generates several different mechanisms to produce smaller droplets and enhance desolvation. These effects combine together to generate a greater number of free ions from the same amount of sample compared to traditional ionization modes, such as electrospray, and typically result in an increase in response across a wide range of compounds.
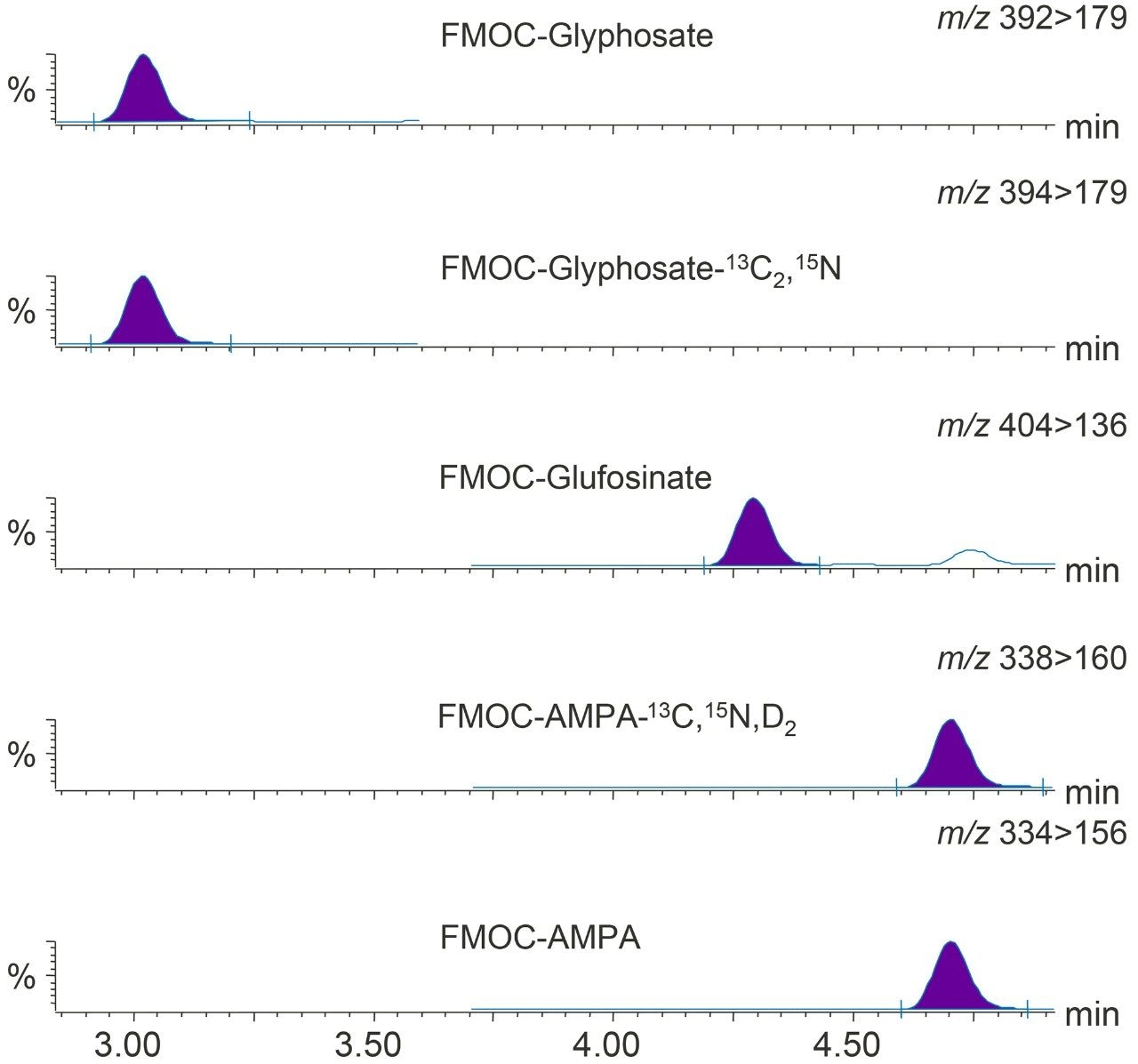
Excellent sensitivity and selectivity was demonstrated by the response for each analyte detected from the analysis of drinking water spiked at 0.02 µg/L (see Figure 2), well below the limits required for drinking, surface, and ground waters. Laboratories are expected to provide methods with lower limits of quantification (LLOQ) of at least one third of the EQS. The sensitivity observed suggests that detection and quantification of all three compounds at much lower concentrations should be possible.
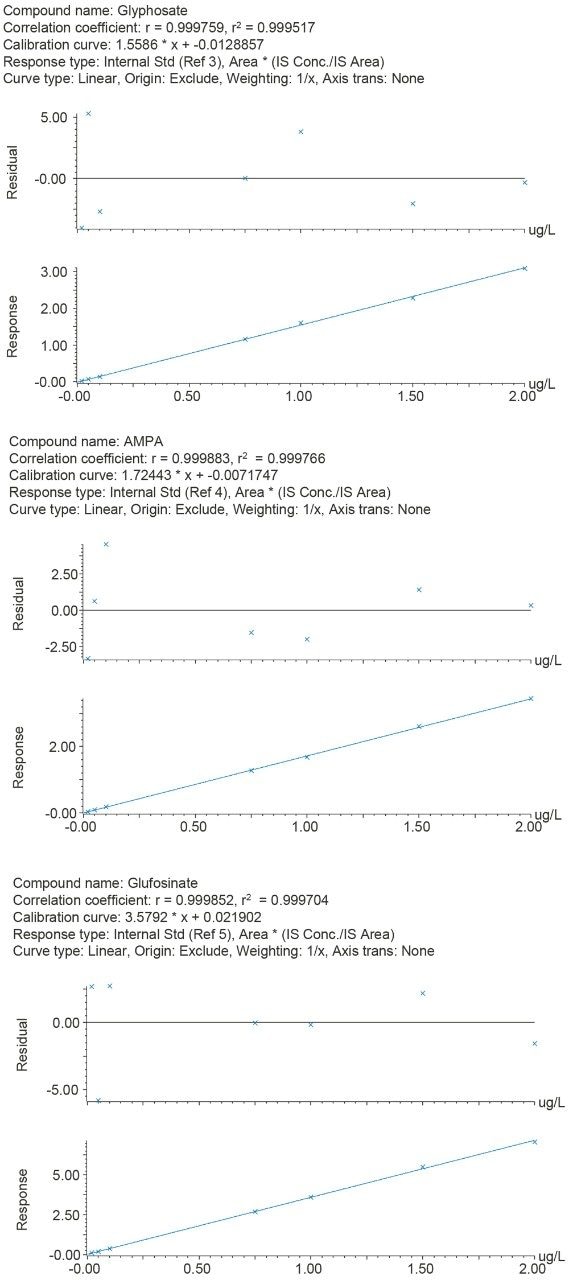
Standard solutions, prepared in drinking water at seven concentrations (0.02, 0.05, 0.10, 0.50, 1.0, 1.5, and 2.0 µg/L), were used for calibration. The response for all three compounds was linear and the correlation coefficients (r2) were >0.999 for all three compounds with residuals of <6% (see Figure 3).
The accuracy of the method was determined from the analysis of spiked water samples. The measured values were compared with the expected values from spiking and found to be within the range 85 to 110% (Table 2). Repeatability of the measurements was also good; e.g. <6% RSD in four different ground water samples spiked at 0.1 µg/L run in duplicate (n=8). Identification criteria, ion ratios, and retention times were all within acceptance tolerances.10
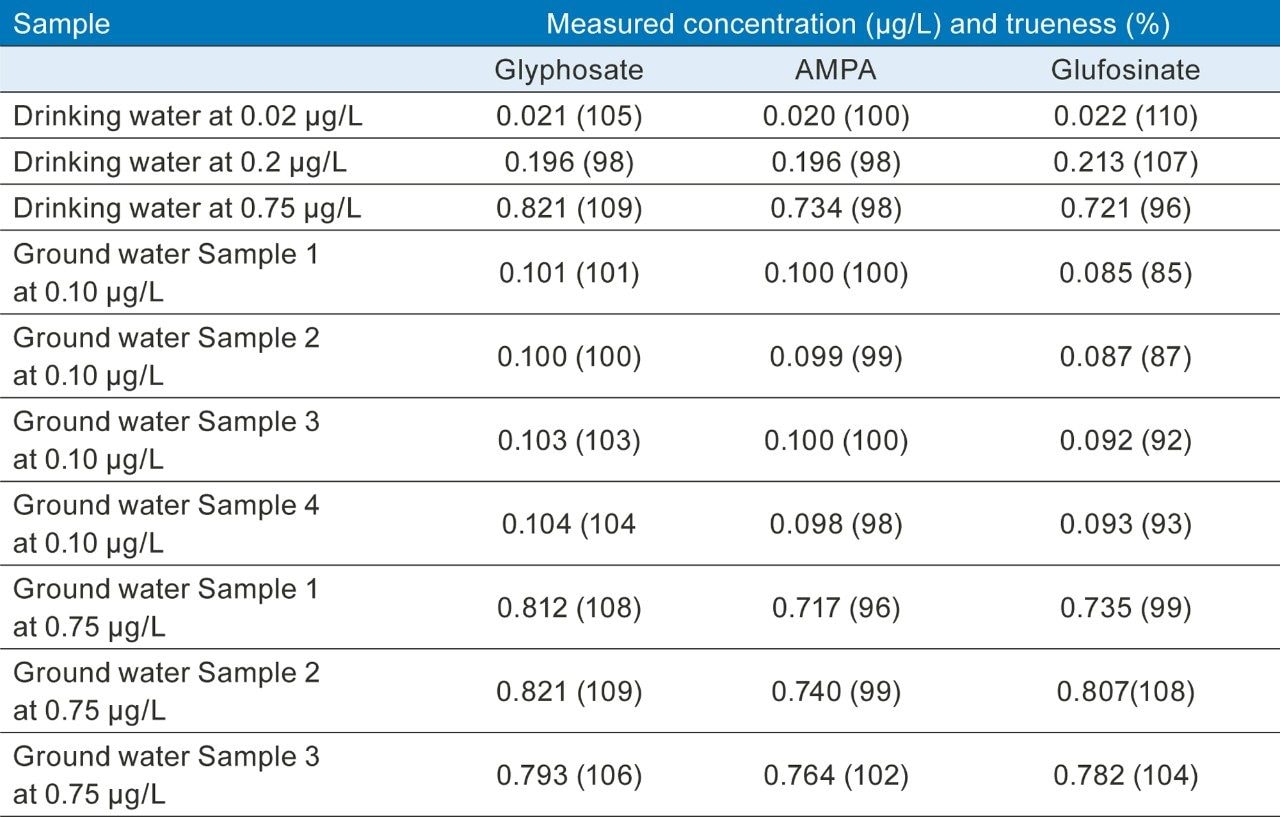
Glyphosate and AMPA were detected in the chromatograms from the analysis of the four water samples (Figure 4), but concentrations were found to be <LLOQ in all but one case; glyphosate (0.021 µg/L) in a sample of ground water.
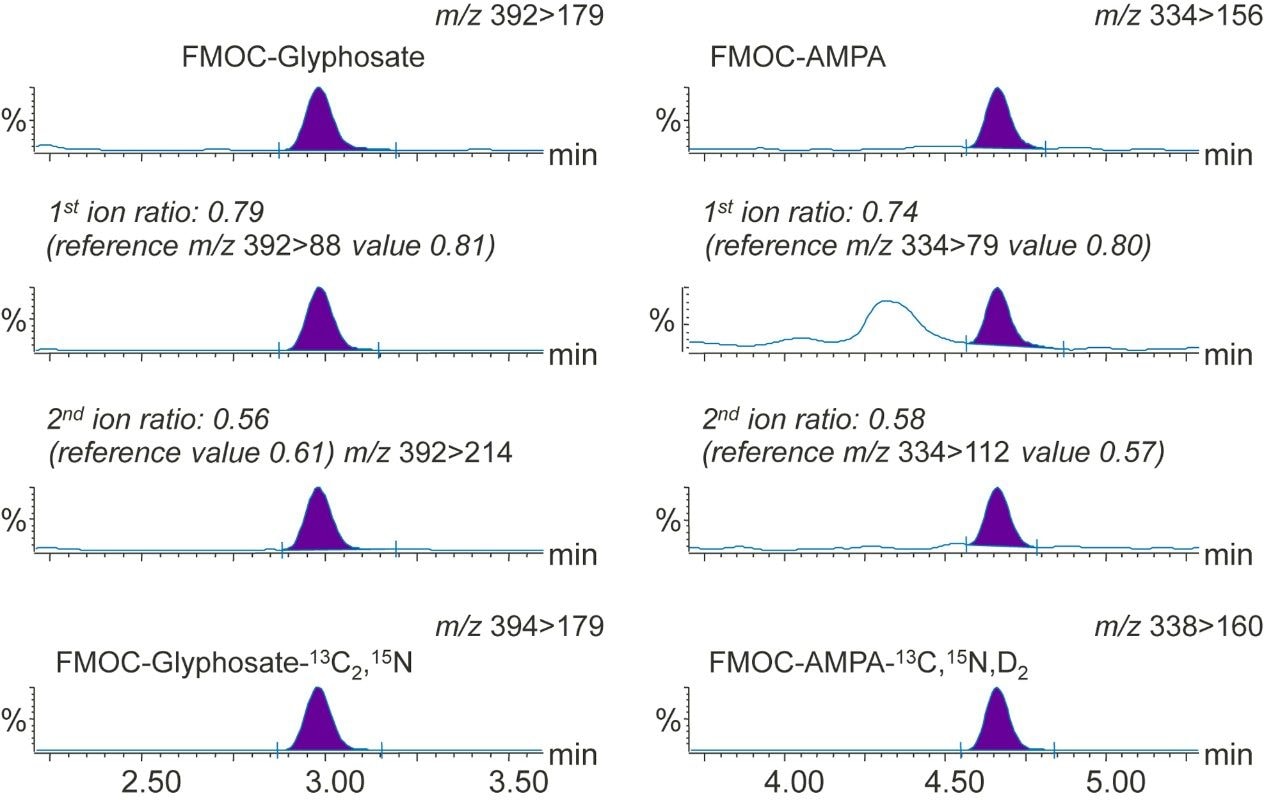
This application note has demonstrated the performance of a method for the determination of glyphosate, AMPA, and glufosinate by UPLC-MS/MS, after derivatization with FMOC, on an ACQUITY UPLC I-Class System coupled to the Xevo TQ-XS MS System. The method is simple, time-saving, and inexpensive, providing fast and reliable quantitation of glyphosate, AMPA, and glufosinate in various types of water samples. The results indicate that this method is suitable for the detection of glyphosate, AMPA, and glufosinate for monitoring purposes. Calibration characteristics, linearity, and residuals were excellent over the concentration range studied. The accuracy of the method was shown to be good, and the method was applied to the analysis of real water samples. Scientists must validate the method in their own laboratories and demonstrate that the performance is fit for purpose and meets the needs of the relevant analytical control assurance system.
The authors gratefully acknowledge Brabant Water, Evides, and Waterleiding Maatschappij Limburg for providing the water samples.
720006246, April 2018