For forensic toxicology use only.
The objective of this current analytical work was to evaluate the performance of Tof-MRM – a targeted mode of acquisition, which is also available on the same HRMS platform and to develop a confirmatory method for the fentanyl class. A secondary aim was to utilize a previously reported novel technique i.e., Threshold Accurate Calibration (TAC), for matrix normalization without the requirement for deuterated internal standards.
High resolution mass spectrometry (HRMS) using quadrupole time-of-flight (QTof) instrumentation is increasingly used within the field of forensic toxicology as a comprehensive screening technique. Typically it is used in a non-targeted mode of data acquisition i.e., Tof-MSE, which provides a highly specific identification based on a combination of precursor and fragment ions generated under low and high-energy conditions, respectively.1-4 The Tof-MSE approach has also been applied recently as the confirmatory step in a dual-definitive workflow protocol for commonly analyzed drug substances in forensic urine drug testing.5
QTof instruments can also be used in targeted acquisition mode, when the goal is to identify and quantify a more limited panel of analytes of interest.6 In recent years, the availability and use of illicit fentanyl and, in particular fentanyl analogues, has become increasingly evident in forensic toxicology. Consequently updated methods, which enable the sensitive detection and confirmation of these emerging substances, are urgently required.
The objective of this current analytical work was to evaluate the performance of Tof-MRM – a targeted mode of acquisition, which is also available on the same HRMS platform and to develop a confirmatory method for the fentanyl class. A secondary aim was to utilize a previously reported novel technique i.e., Threshold Accurate Calibration (TAC), for matrix normalization without the requirement for deuterated internal standards.7-9
Reference material for the fentanyls (fentanyl, norfentanyl, and all analogues) were obtained from Cerilliant and/or Cayman Chemical at a concentration of 1 mg/mL. A mixed fentanyl stock solution was prepared by dilution in methanol, to give a concentration of 10 μg/mL, and stored at -20 °C until use.
A TAC spiking solution was prepared by dilution of the mixed fentanyl stock with water to give a concentration of 30 ng/mL. Water was used as a corresponding blank spiking solution.
Analyte-free urine was enriched with the mixed fentanyl stock solution to yield a single calibrator at a concentration 2 ng/mL. A lower limit of detection (LLD) control was prepared at 0.8 ng/mL urine.
Quality control samples were prepared by enriching analyte-free urine with a mixed stock of fentanyls from a separate source, to yield the following QCs: 1.5, 3.0, 10 ng/mL urine.
Authentic samples were obtained from routine casework.
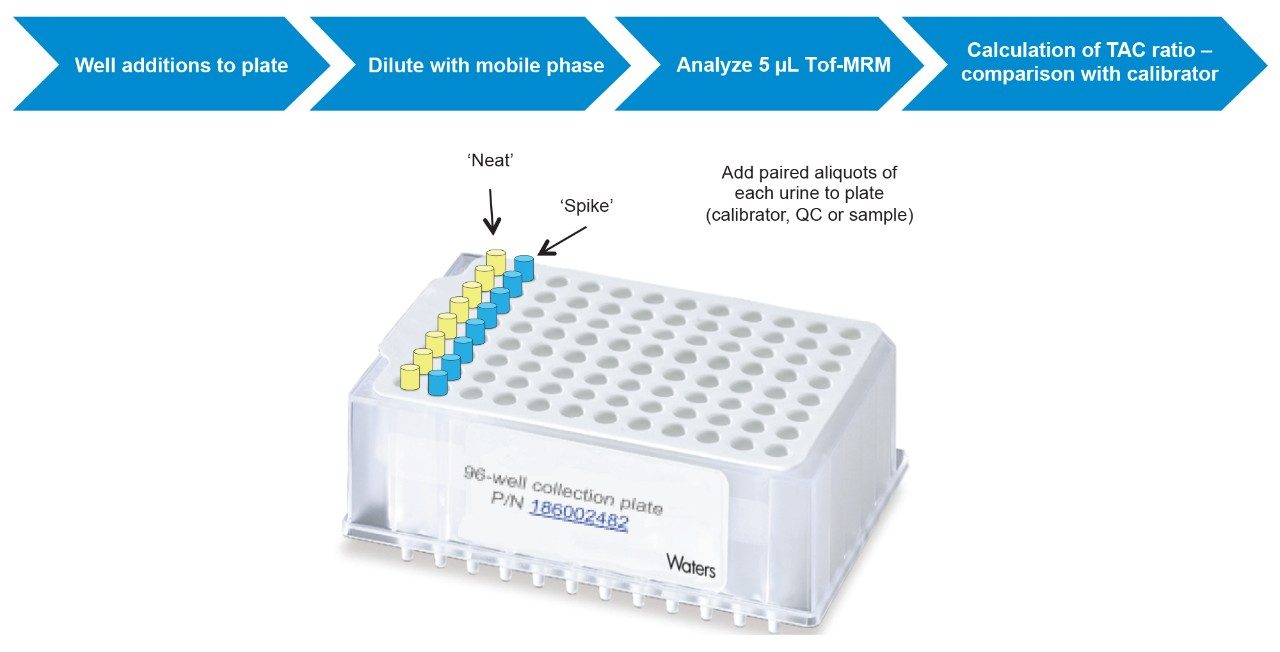
Fifty microlitres of each sample (calibrator, control or case urine) were added to duplicate, adjacent wells of a 96-well plate for analysis of ‘neat’ and ‘spiked’ samples (Figure 1).
Fifty microlitres of the TAC spiking solution was added to all ‘spiked’ wells and 50 μL of blank spike was added to corresponding ‘neat’ wells.
After addition of 500 μL mobile phase (87% MPA:13% MPB) to all wells, the plate was transferred to the ACQUITY UPLC autosampler for the analysis of 5 μL of sample using UPLC-Tof-MRM.
|
LC system: |
ACQUITY UPLC I-Class (FTN) |
|
Column: |
ACQUITY UPLC HSS C18 100 Å, 1.8 μm, 2.1 mm × 150 mm (p/n 186003534) |
|
Column temp.: |
50 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
5 μL |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
5 mM Ammonium formate pH 3.0 |
|
Mobile phase B: |
0.1% Formic acid in acetonitrile |
|
Gradient program: |
Table 1 |
|
MS system: |
Xevo G2-XS QTof |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
0.8 kV |
|
Cone voltage: |
25 V |
|
Desolvation temp.: |
500 °C |
|
Desolvation gas flow: |
1000 L/hr |
|
Cone gas: |
20 L/Hr |
|
Acquisition mode: |
Tof-MRM (Table 2) |
UNIFI Scientific Information System was used for instrument control and data processing.
A previously-described approach (Threshold Accurate Calibration; TAC)1-3 was employed in this study to prepare the samples and to normalize matrix effects without the use of deuterated internal standards.
Briefly, calibrator, QC and unknown samples were analyzed without (‘neat’), and with (‘spiked’), addition of a cut-off amount of reference analytes (Figure 1).
The TAC ratio of ‘neat’ to ‘spiked’ peak-area response was determined for each specimen and compared with the ratio obtained for the urine calibrator containing drugs at the cut-off threshold concentration (in this assay, 2 ng/mL) for a simplified qualitative presentation of results e.g.:
TAC ratio = ‘Neat’ peak-area response /‘Spiked’ peak-area response – ‘Neat’ peak-area response
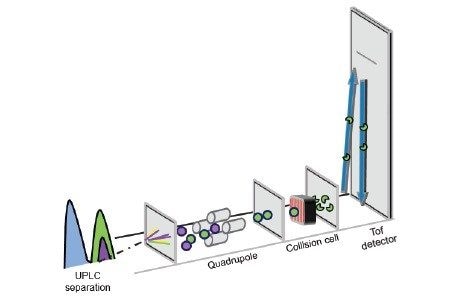
Time-of-flight-mass spectrometers (Tof-MS) are typically used in a non-targeted acquisition mode such as MSE to facilitate broad forensic screening.4-6 Although this mode already provides a sensitive assay with limits of detection in the low ng/mL range, the same instrument can also be used in an alternative, mode e.g., Tof-MRM (Figure 2), this targeted mode can offer further increases in sensitivity; indeed, enhancements ranging from 2 to 200-fold have been reported.7 Improved sensitivity can be useful where analytes are likely at very low concentrations and/or where simplified sample preparation techniques such as dilution-only protocols, are being utilized.
In this study, Tof-MRM was applied to diluted urine samples urine samples to analyze fentanyls – due to their high potency, these substances are often encountered in samples at low, or sub-ng/mL concentrations.
An initial evaluation of Tof-MRM mode, using calibrators, revealed an increased sensitivity over Tof-MSE; increases ranged from 5 to 20-fold. Owing to the aim to use a simple sample preparation protocol (effectively a 10-fold dilution of the sample) the targeted acquisition mode was utilized for the remaining study.
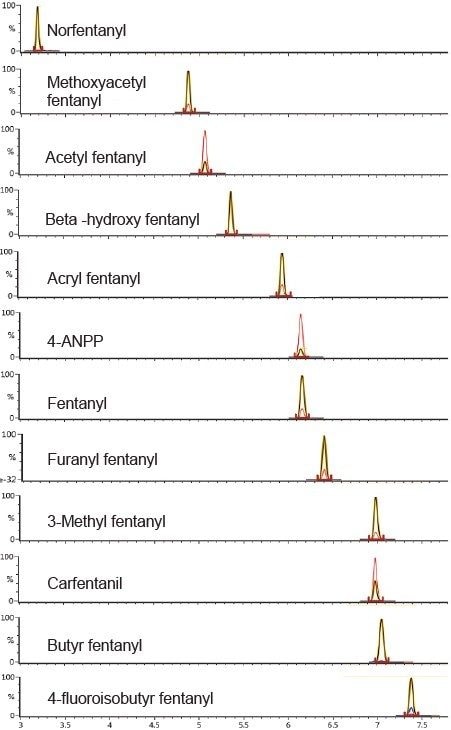
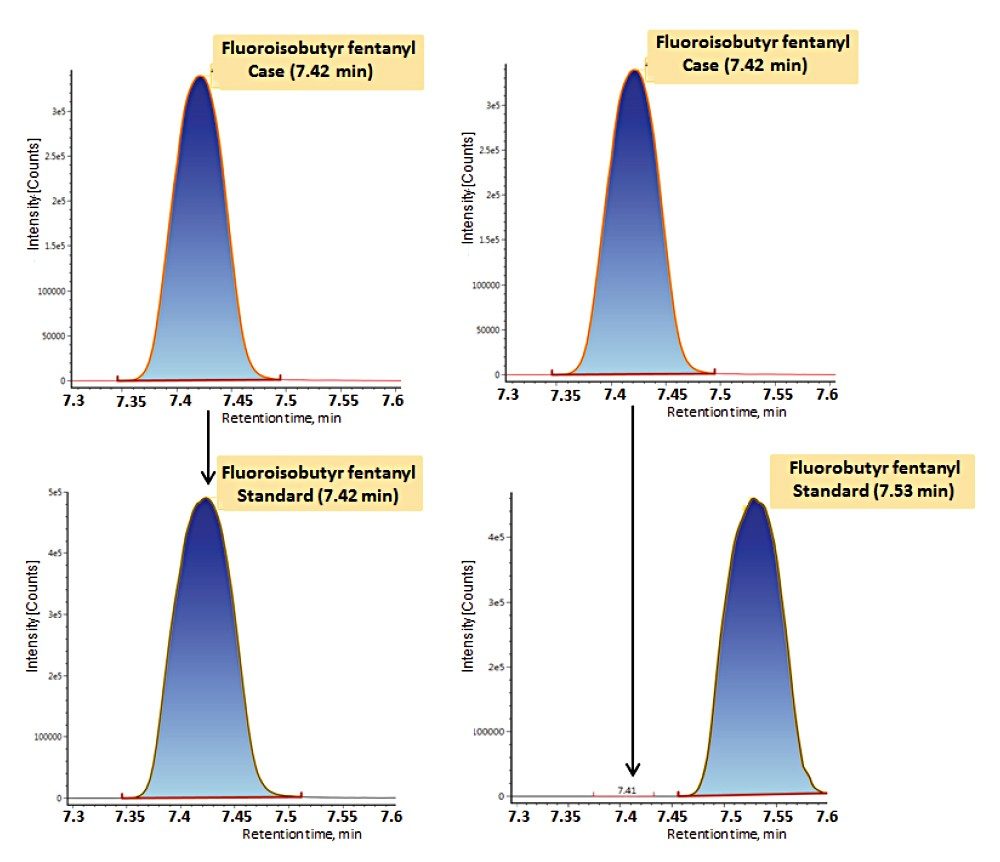
Samples were prepared using a simplified in-well sample protocol (Figure 1) and subsequently analyzed using a 15 minute chromatographic separation (Table 1), combined with optimized dual-transition Tof-MRM monitoring (Figure 3, Table 2). The method was highly sensitive and also permitted differentiation of structural isomers (Figure 4).
Validation studies were conducted over 17 analytical runs and were designed in accordance with New York State Department of Health guidelines. The innovative TAC approach normalized the matrix effect and allowed consistent, threshold-accurate detection of all fentanyls investigated. Acceptable precision and accuracy was demonstrated for analyte concentrations around the cut-off i.e., at 0.8, 1.5, 3.0, and 10.0 ng/mL (Figure 5).
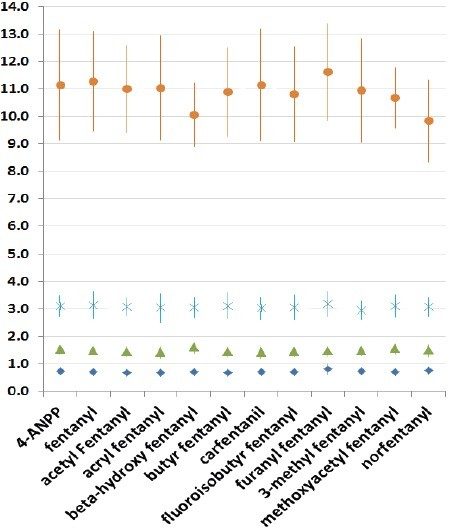
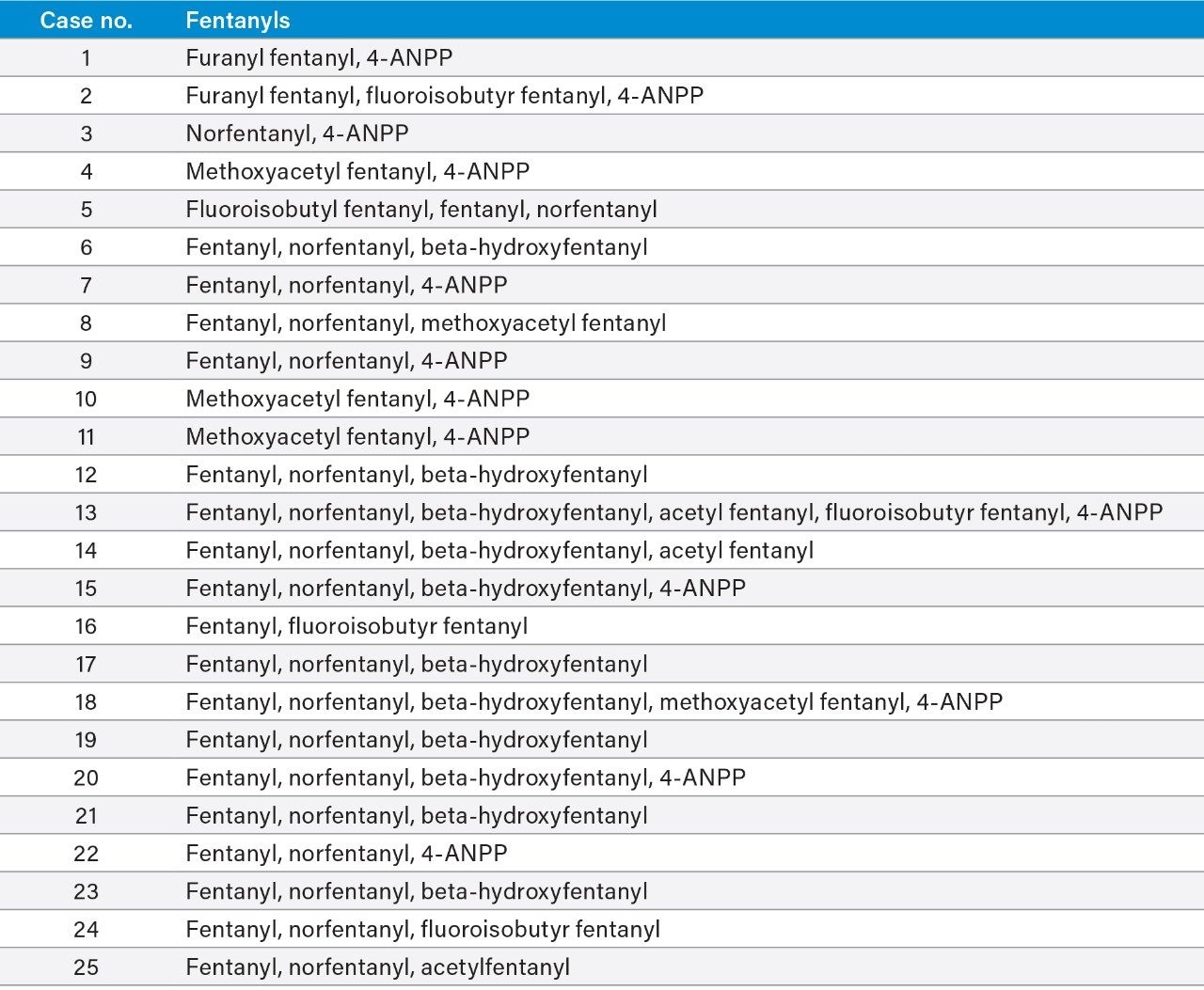
The confirmatory method was applied to 25 forensic case samples that had been also analyzed by a separate, fast, UPLC-MS/MS based screening method and the Tof-MRM demonstrated 100% concordance with the independent screen.8 All case findings are summarized in Table 3, with fentanyl, norfentanyl, beta-hydroxy fentanyl, and 4-ANPP among the most commonly found. 4-ANPP is an intermediate, used in the manufacture of fentanyls and as such can be found as an impurity in fentanyl preparations; it is also understood to be a metabolite of fentanyl and some of the analogues including furanyl fentanyl, acetyl fentanyl, and acryl fentanyl.
This application note describes a confirmatory method for use in forensic toxicology.
In line with previous reports, Tof-MRM provided increased sensitivity over the non-targeted Tof-MSE approach.
The method utilizes some innovative approaches to produce a simple, yet accurate and precise qualitative method for the analysis of fentanyl and fentanyl analogues in urine.
The TAC approach, resulted in an accurate qualitative confirmation without the requirement of deuterated internal standards, which may not always be available, particularly for newer drug analogues.
The TAC approach also means that the method is adaptable and can be readily updated as new fentanyl analogues emerge.
720006515, March 2019