
A new technique, QuEChERS, standing for Quick, Easy, Cheap, Effective, Rugged and Safe, is readily accepted by both the AOAC International and the Committee of European Normalization (CEN) for the pesticide residues in foods and agriculture products. Waters DisQuE Dispersive Sample Preparation Kit contains conveniently-packaged centrifuge tubes with pre-weighed sorbents and buffers designed for use with the AOAC official QuEChERS methods.
The DisQuE Dispersive Sample Preparation Kit contains: DisQuE extraction (tube 1), a 50 mL centrifuge tube containing 6 g of anhydrous magnesium sulfate and 1.5 g of anhydrous sodium acetate, and DisQuE clean-up (tube 2), a 2 mL centrifuge tube containing 50 mg of primary secondary amine (PSA) sorbent and 150 mg of anhydrous magnesium sulfate.
The typical procedure consists of two steps: a well homogenized aqueous sample is first extracted by acetonitrile in the presence of high amount of desiccant (anhydrous magnesium sulfate) and buffer (acetic acid/sodium acetate). After centrifugation the organic extract is further cleaned up by dispersive solid-phase extraction (d-SPE), typically using primary secondary amine (PSA) sorbent. PSA sorbent, combined with MgSO4, has been demonstrated to be very effective in removal of organic acids, excessive water, and other components. The extract is subs quently analyzed by either GC or LC (or both), often coupled with mass spectrometry (MS) depending on the pesticides of interest.
The fruit sample is chopped into small portions, and then pulverized by a food blender until it reaches homogeneous texture. Transfer 15 g of the homogenized fruit sample into the extraction tube 1, then add 15 mL of 1% acetic acid in acetonitrile. Shake the tube vigorously for 1 minute and centrifuge at 1500 rcf for 1 minute. Transfer 1 mL of the upper layer extract from tube 1 to clean-up tube 2. Shake tube 2 vigorously for 1 minute and centrifuge at 1500 rcf for at least 1 minute. The extract is subsequently analysed by either GC-MS or LC-MS depending on the pesticides of interest.
|
LC-MS system: |
Waters ACQUITY UPLC System with Quattro Premier XE Mass Spectrometer |
|
Columns: |
ACQUITY UPLC BEH 1.7 μm C18, 2.1 x 100 mm |
|
Mobile phase: |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
|
Gradient: |
Indicated in Figure captions |
|
Flow-rate: |
0.5 mL/min |
|
Injection: |
1 μL |
|
Temperature: |
40 °C |
|
Detection: |
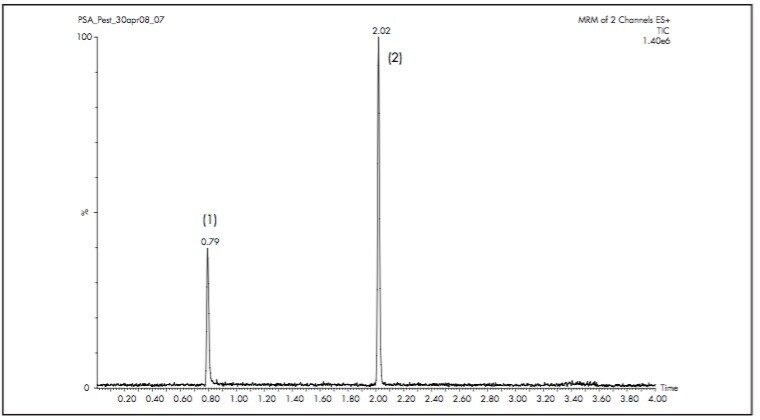
Multiple reaction monitoring (MRM) transitions, 202→175 for thiabendazole and 216→174 for atrazine |
|
GC-MS System: |
Agilent 6890N GC System with a Waters Quattro micro GC Mass Spectrometer |
|
Columns: |
Restek Rtx-5MS, 30 x 0.25 mm i.d., 0.25 μm df |
|
Carrier Gas: |
Helium |
|
Temp. Program: |
Initial temp at 80 °C, hold for 1 min, 10 °C/min to 320 °C, hold for 5 min |
|
Flow-Rate: |
1.0 mL/min |
|
Injection: |
Split/splitless mode with 0.5 min purge time and 1 μL injection |
|
Detection: |
Selected Ion-Recording mode (SIR) with these compounds ion mass (m/z) monitored, phenylphenol 170, 141 atrazine 200, 173 chlorpyrifos methyl 286, 109 DDD 235, 165 ethion 231, 153 cylohalothrin 197, 181 |
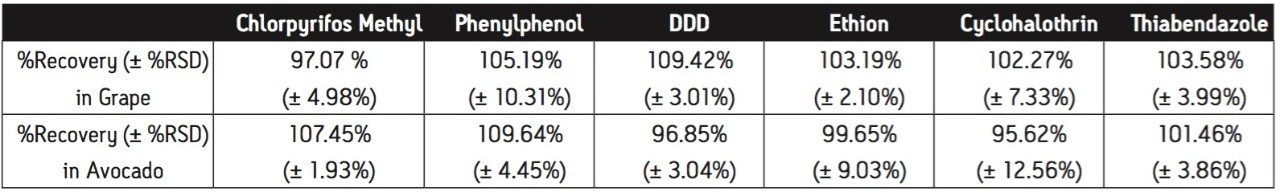
Pesticides were fortified into two fruit matrices: grape and avocado. Atrazine was employed as the internal standard (IS) for both GC-MS and LC-MS analysis. A spiking solution of the target pesticides was prepared at 50 μg/mL of each compound. 40 μL of the spiking solution was added to 10 mL of sample extract to give a nominal concentration of 200 ng/mL for each pesticide. The same amount of pesticides was spiked to the extracts either prior to the SPE procedure or after the SPE cleanup. The recovery of each pesticide was calculated by comparing the concentrations in the samples where the pesticides were spiked prior to the SPE procedure to those where the pesticides were spiked after the SPE cleanup. The recovery results in avocado and grape are summarized in Table 1. The % recoveries of pesticides in grape are ranged from 97% to 109%. The pesticides are recovered in avocado ranging from 96% to 110%. There was no significant loss of pesticides due to the SPE clean-up procedure using PSA sorbent.

The DisQuE dispersive sample preparation kit is convenient to use. The procedure is simple and straightforward to follow and is very effective for removing matrix interferences commonly associated with fruit matrices without significant losses of pesticides. This procedure is applicable to most basic and neutral pesticides. Acidic pesticides will be retained by the PSA sorbent in Tube 2. The acidic pesticides could be analyzed directly by LC-MS after diluting the extract with water, or the extract could be subject to an alternative cleanup procedure.
720002755, August 2008