This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates to provide a system for direct sample introduction of lubricating oil formulations into a mass spectrometer for material characterization without sample preparation.
The ability of ASAP to generate ions by both charge transfer and proton transfer has enabled the ionization of a wide range of compounds of varying polarity.
The analysis of complex mixtures in multi-component matrices is a recurring requirement for industrial mass spectrometry laboratories.The full analysis of particularly complex samples may require a strategy that involves several techniques, such as GC-MS and LC-MS. Typical of this type of challenge is the analysis of lubricant formulations. These formulations generally consist of a mineral oil base with anti-oxidants and other performance additives. An oil/additives package of this type contains compounds of widely differing volatility and polarity that provide a significant challenge to analysts.
ASAP (Atmospheric Solids Analysis Probe) developed by McEwen et. al.1 is a useful tool for the rapid and direct analysis of volatile and semi-volatile solid and liquid samples using atmospheric pressure ionization. The ASAP technique is capable of ionizing low polarity compounds not amenable to Electrospray Ionization (ESI) and Atmospheric Pressure Chemical Ionization (APCI) with high sensitivity. It can also be used for the analysis of complex samples without the need for any sample preparation. The use of ASAP as a sample inlet for Time-of-Flight mass spectrometry (ToF MS) allows for the analysis of complex mixtures, such as lubricating oil, to determine the exact mass of the functional additives.2
An ASAP inlet was used to introduce samples into a Waters SYNAPT G2 HDMS System. Samples were loaded directly onto a sealed glass melting point tube without any sample preparation and vaporized in a stream of heated nitrogen. The sample in the gas phase was ionized in proximity to a corona discharge needle. Ions were then passed from the atmospheric pressure region into the mass spectrometer. Use of APCI allows for evaluation of chemical modifiers, such as methanol, to influence the ionization mechanism, as shown in Figure 1.
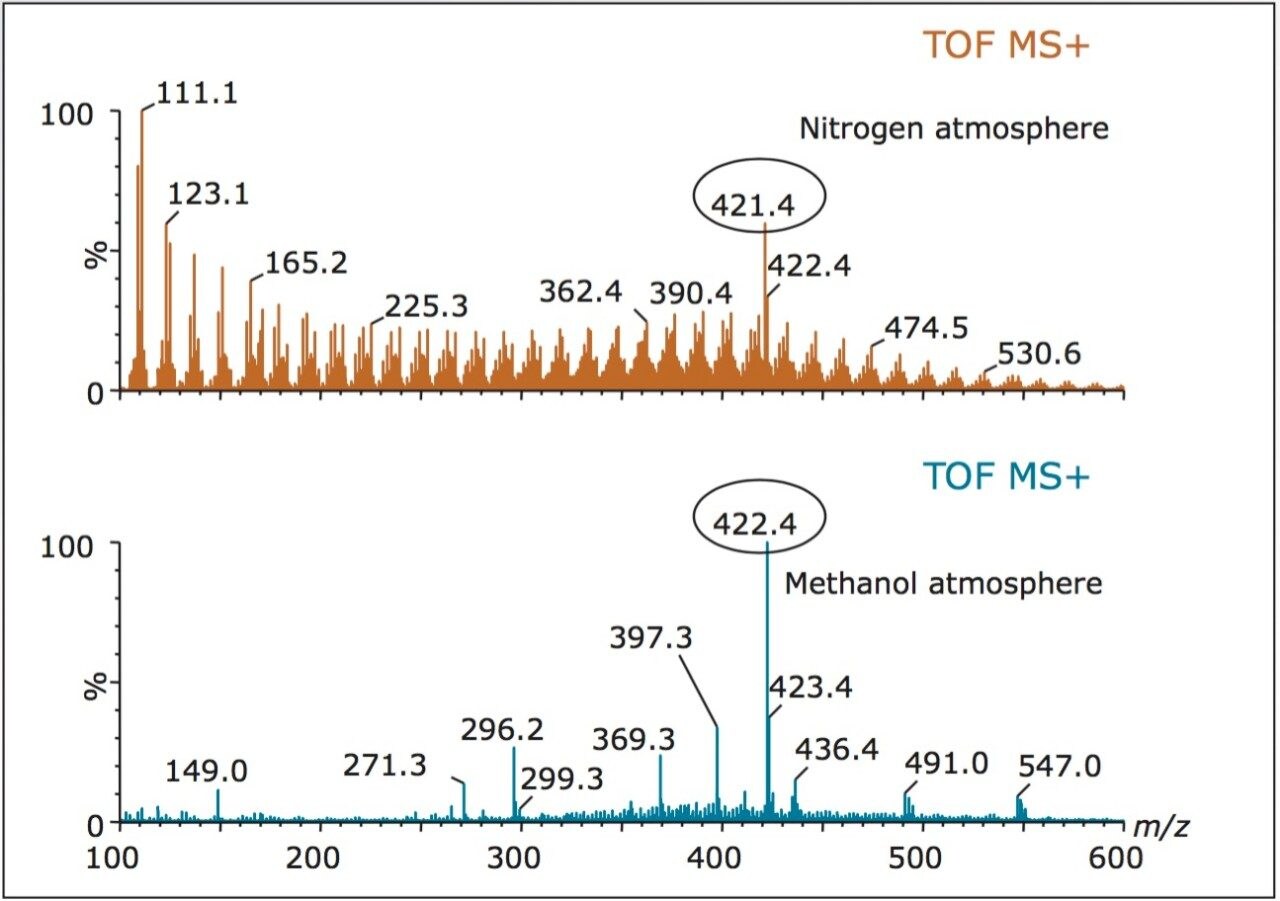
This example shows the ASAP analysis of a lubricating oil before and after the addition of methanol to the source region. The addition of the methanol clearly shows the enhancement of the amine antioxidants, which have a higher proton affinity, resulting in the formation of the protonated molecules. The dry nitrogen atmosphere favors the formation of radical cations of the mineral oil by charge transfer. The peak at m/z 421 (M+) in the dry nitrogen atmosphere and m/z 422 ([M+H]+) in the methanol atmosphere are di-nonyl diphenylamine, a commonly used antioxidant. Although the di-nonyl diphenylamine can also be observed as a radical cation at m/z 421 in the spectrum obtained under dry nitrogen atmosphere conditions, the introduction of the chemical modifier allows for analyte discrimination.
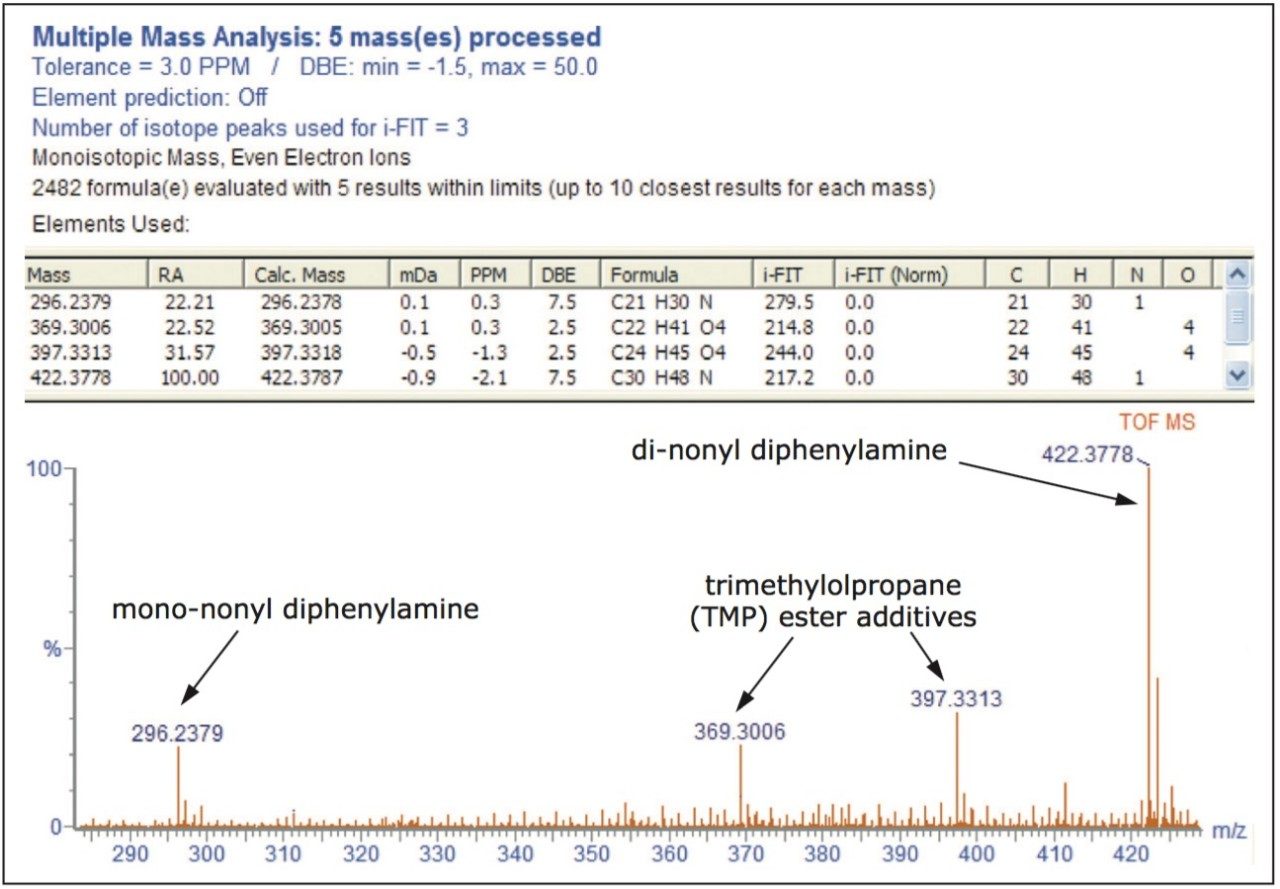
The elemental composition report for the additives apparent in the methanol atmosphere spectrum is shown in Figure 2. The results indicate the presence of trimethylolpropane (TMP) ester additives at m/z 369 and 397, while the peaks at m/z 296 and 422 are mono and di-nonyl diphenylamine respectively. These are commonly used antioxidants.
720004025, September 2011