This work demonstrates the benefits of the Regulated Bioanalysis System Solution from Waters, which combines solid phase extraction methodology, UPLC chromatography, and an advanced tandem quadrupole MS. The system is used for the development of a highly sensitive method for quantification of budesonide in plasma that also offers high selectivity and throughput.
Addresses the challenges including instrutment’s high throughput capability, robustness, ability to address upcoming analytical demands, and ability to address regulatory guidelines.
Budesonide is a glucocorticoid steroid used for the treatment of asthma and noninfectious rhinitis (including hay fever and other allergies), and also for treatment and prevention of nasal polyposis. In addition, it is used for Crohn’s disease (inflammatory bowel disease). Budesonide, in comparison with prednisolone, has been associated with fewer bone density losses and, unlike other corticosteroids, has little influence on hypothalamic-pituitary-adrenal axis, which also limits the need of tapering before discontinuation. Overall, Budesonide has a lower incidence of systemic manifestations than similar medications.
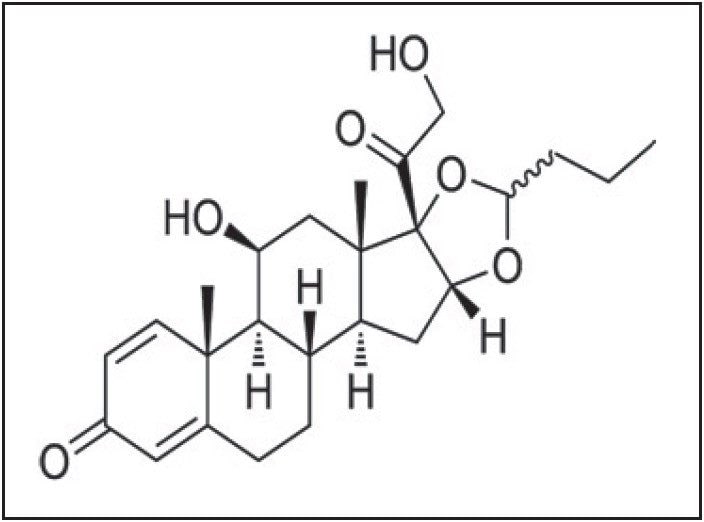
Estimating Budesonide at low levels in complex matrices, such as human plasma, is a challenge due to the extremely low circulatory levels in plasma (10% bioavailability) and high affinity to bind proteins. In this application note, we successfully report an LC-MS/MS analysis of Budesonide with an LLOQ of 2 pg/mL.
The samples were isolated using solid phase extraction with a Waters Sep-Pak C18 (1 cc, 50 mg) Cartridge. A 500 μL aliquot of plasma was precipitated with zinc sulfate, then diluted with ammonia and loaded onto the SPE cartridge previously conditioned with organic solvent and water. The plasma solution was then washed with water followed by an organo-aqueous solution and elution in solvent. The eluted samples were evaporated to dryness and reconstituted in 50% acetonitrile. Zinc sulfate and acetonitrile were purchased from Fluka (MO, USA).
The chromatographic method utilized an ACQUITY UPLC System with an ACQUITY UPLC BEH C18 150 mm, 1.7 μm, 2.1 x 150 mm Column; this provided excellent resolution for the Budesonide analyte from the endogenous components in the samples. Budesonide eluted at 2.71 min with a peak width of 12 s at the base. The data illustrates both the blank signal and the signal obtained from the lower limit of quantification (LLOQ) of Budesonide in human plasma, shown in Figure 2.
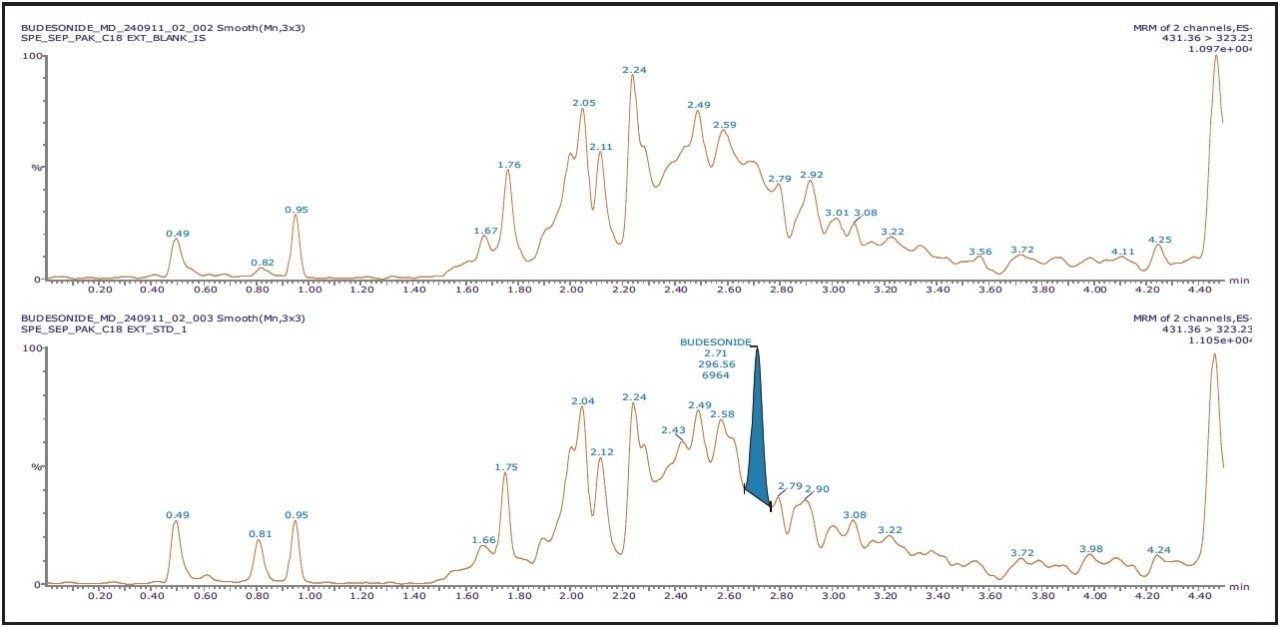
As seen in Figure 2, the retention time of Budesonide does not interfere with the background; the signal corresponding to the analyte of interest can be easily observed even at the LLOQ level. The Xevo TQ-S MS is equipped with a novel StepWave ion guide, which when combined with the high-resolution chromatography obtained from the ACQUITY UPLC System, results in successful completion of extremely sensitive applications to be performed with high reproducibility. As observed from Figure 2, the benefits of Xevo TQ-S along with outstanding sensitivity observed from UPLC allows detection of Budesonide at a concentration of 2 pg/mL, with a signal-to-noise ratio of 35:1.
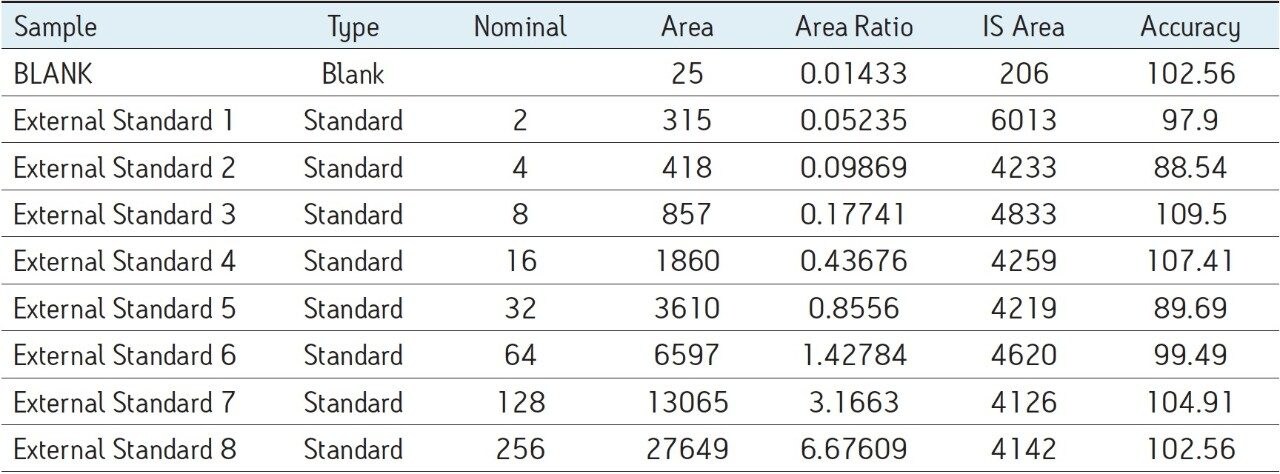
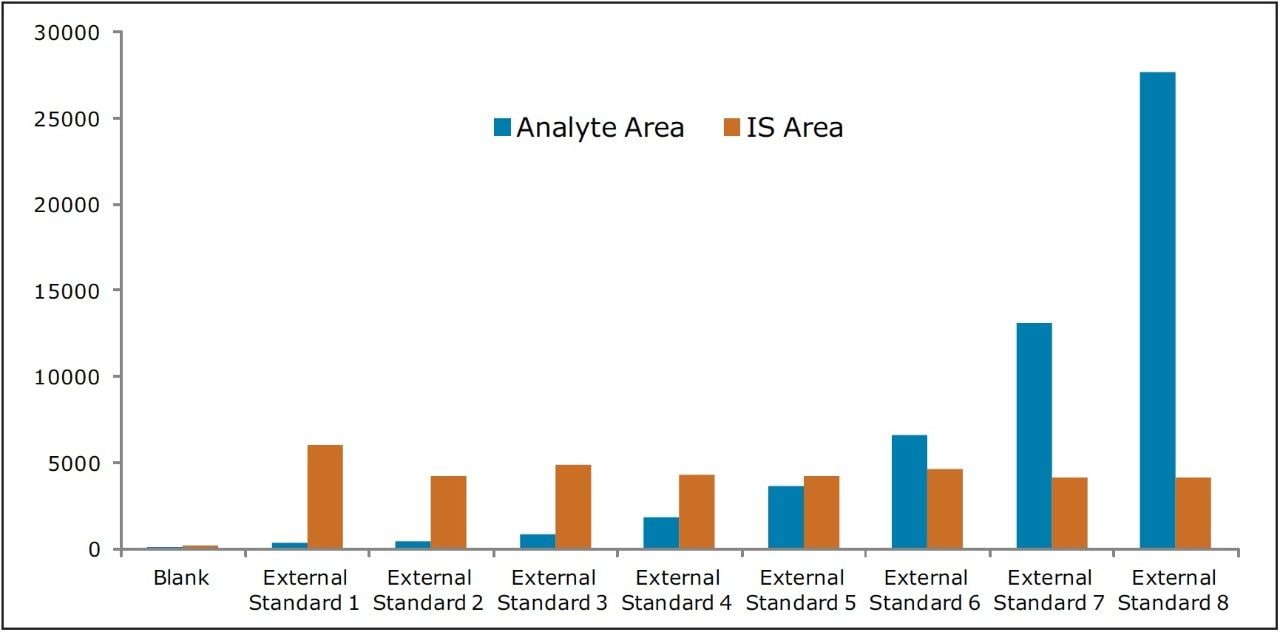
The assay in this report showed linear calibration over the range of 2 to 256 pg/mL with an excellent r2 value of 0.992, shown in Table 1 and Figure 3. The back-calculated concentration of the standard was found to be within ±12% of the nominal concentration (Table 1), and an excellent degree of accuracy was achieved for each sample. This assay was performed with a 7 min injection-to-injection time scale highlighting the capability of Waters Bioanalysis System solution to deliver highly sensitive and specific results while maintaining desired precision and high throughput value.
Recovery of the analyte and internal standard (IS) was performed by comparison of extracted QC samples against six post-extracted samples and was found to be approximately 75% at LQC, MQC, and HQC levels for both analyte and IS (Figure 4 and Table 2). The %CV for repeat batches were found to be within 10% of LLOQQC and varied between 1% to 3% for all QC levels.
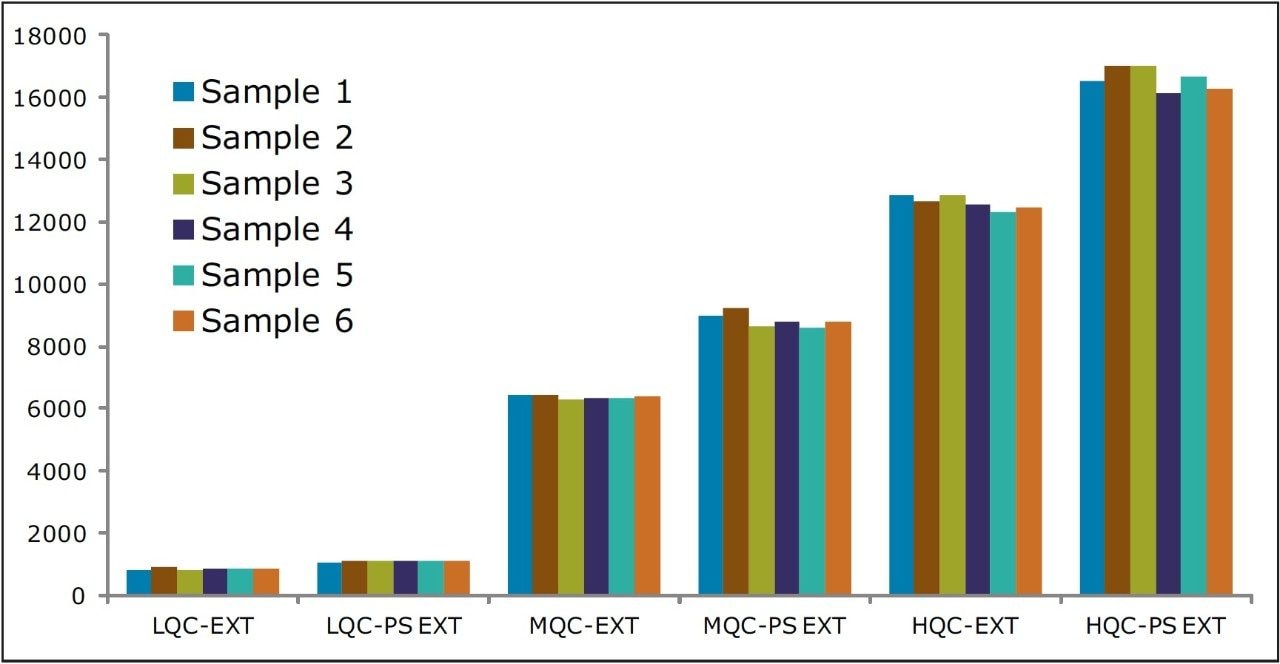
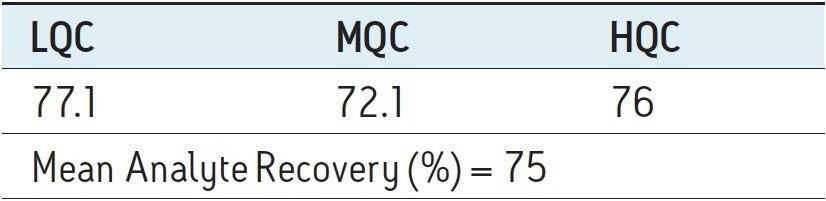
As can be observed from the data shown in Figure 4, the analyte recovery values for the six samples for all three concentration levels (LQC, MQC, and HQC) did not vary significantly. In addition, as detailed in Table 2, the mean analyte recovery for the three concentration ranges was well within acceptable limits.
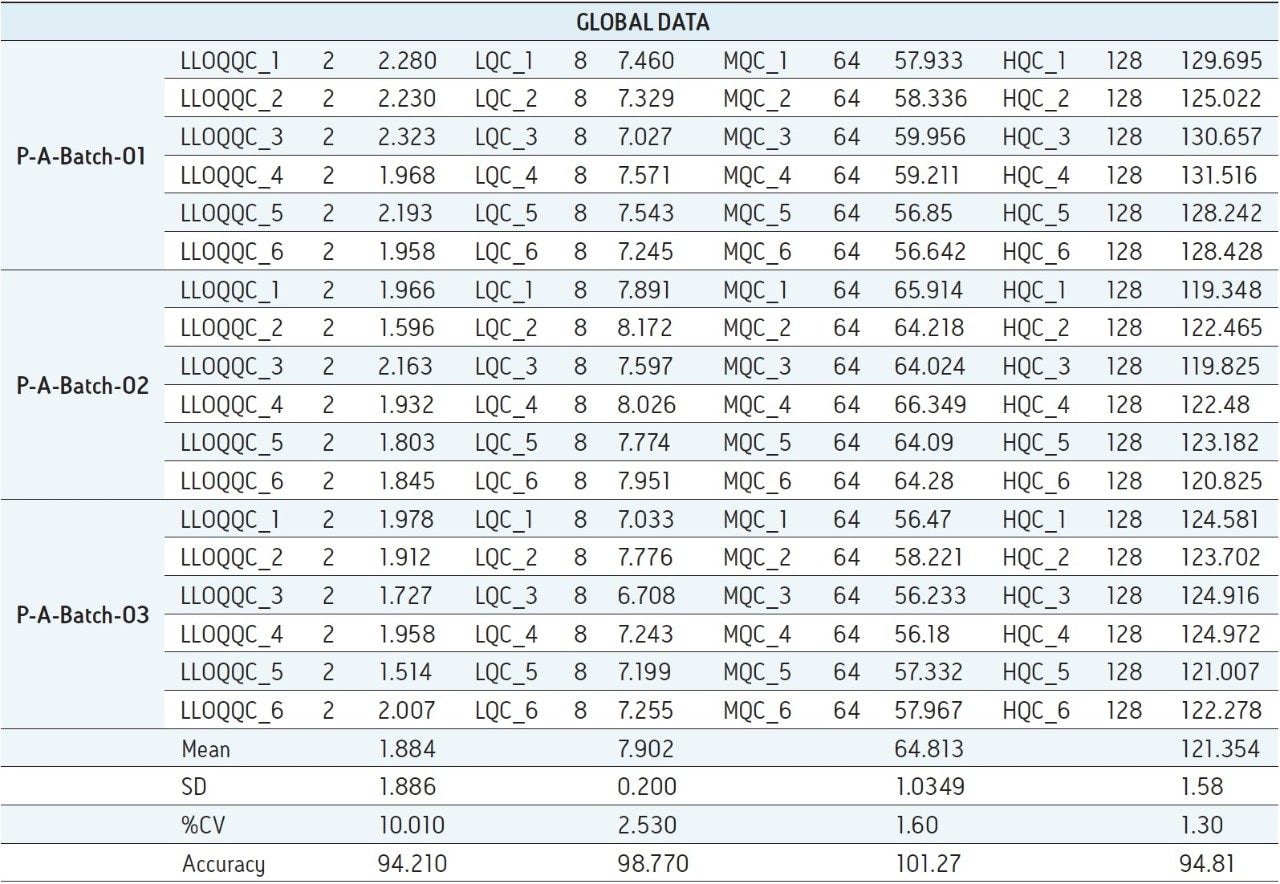
For a comparison of samples within the global batches, three separate batches were prepared with six samples in each batch for LLOQQC, LQC, MQC, and HQC concentration levels. The data showed excellent agreement between the six samples in all the three batches (Table 3). The mean accuracy obtained for all the sample levels was found to be > 94% for every concentration (Table 3). This outstanding quality of data was achieved using the Regulated Bioanalysis System solution.
Budesonide is a glucocorticoid steroid used for the treatment of asthma and rhinitis. However, due to the extremely low circulatory levels in plasma (10% bioavailability) and high affinity to bind proteins, estimation of Budesonide at low levels in complex matrices like human plasma is a challenging task. In this application note and with the successful use of Waters Regulated Bioanalysis System solution, we report an LC-MS/MS analysis of Budesonide with an LLOQ of 2 pg/mL. In addition to achieving the sensitivity demands, there are several other challenges that affect today’s biopharmaceutical companies. These challenges include the instrutment’s high throughput capability, robustness, ability to address upcoming analytical demands, and ability to address regulatory guidelines. The results detailed in this application note successfully address each of these challenges.
720004198, January 2012