This application note showcases the capabilities of the Xevo TQD Mass Spectrometer, ACQUITY UPLC H-Class System, sample preparation, and column chemistries to achieve a sensitive LC-MS/MS method for the detection and quantification of exenatide in plasma.
Bioanalysis of biotherapeutics has attained increased interest in the bioanalysis community in recent years. As required for all drug candidates, determination and quantification of peptide therapeutics should be performed at low concentrations while addressing all regulatory concerns, ensuring reproducibility, and maintaining method robustness with high throughput value. In addition, it is advisable for these studies to be conducted with minimal financial and other resource investment.
The use of peptides and proteins as therapeutic agents has increased significantly in recent years. The bulk of the drug pipeline in most major pharmaceutical companies is now comprised of peptides and proteins. With increased focus on biotherapeutics, the use of LC-MS for the quantitative analysis of proteins and peptides has gained substantial interest due to its accuracy, dynamic range capability, and speed of method development.
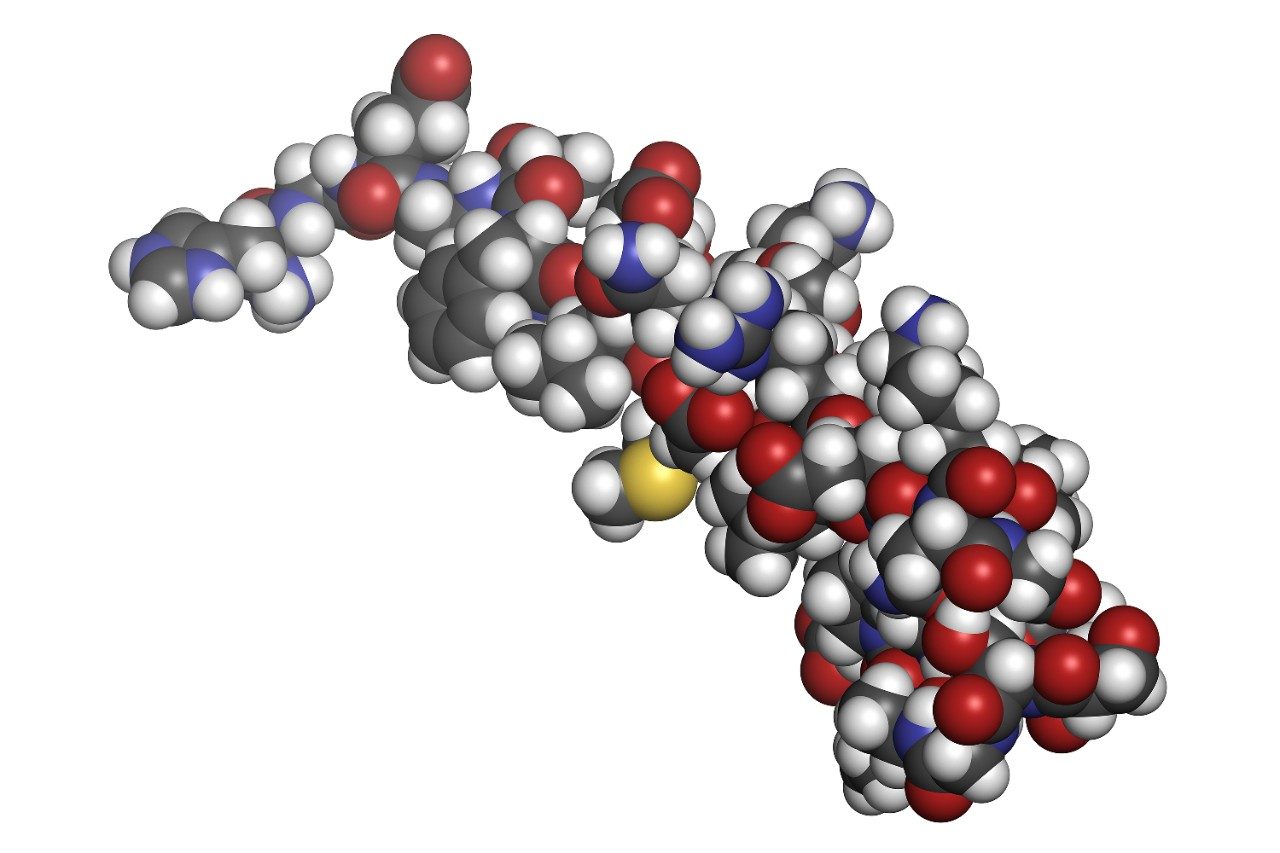
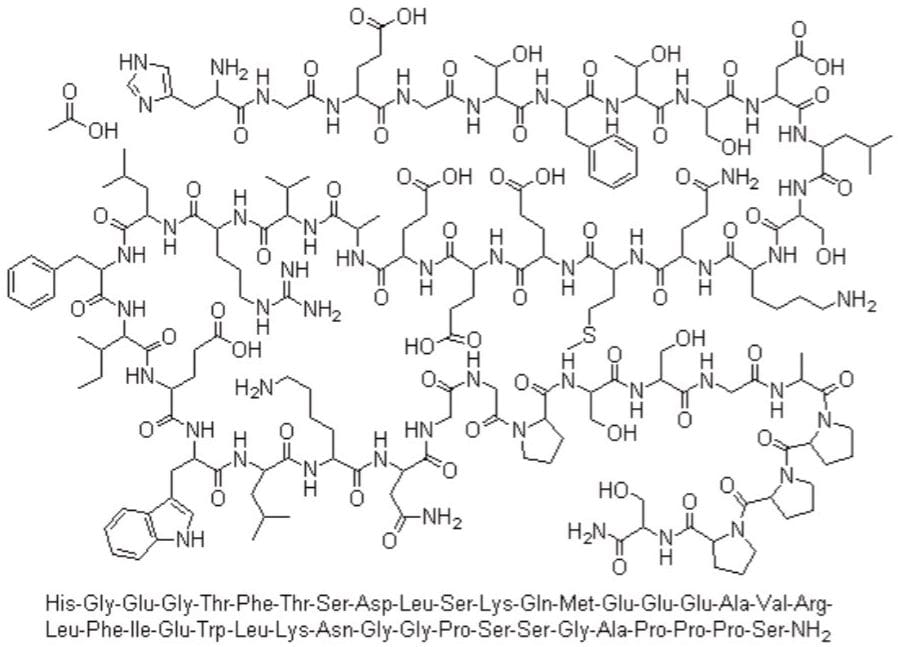
Exenatide, shown in Figure 1, is a large therapeutic peptide that is a synthetic version of Exendin-4, a hormone found in the saliva of the Gila monster. It is a 39-amino-acid peptide with a molecular weight of 4186.6 Da. Exenatide has been approved for the treatment of diabetes mellitus type 2 as an adjunctive therapy marketed as Byetta (Amylin Pharmaceuticals). Exenatide enhances glucose-dependent insulin secretion by the pancreatic beta-cell; thereby, regulating glucose metabolism and insulin secretion. It slows the emptying of the gastric system, increases satiety, reduces appetite, and lowers liver fat content.
Traditionally, the plasma concentrations of exenatide have been measured by ligand-binding assays, such as immunoenzymetric assays used for pharmacokinetic studies. Developing antibodies and assays for ligand-binding assays is very time-consuming, cost prohibitive, and lacks the precision and accuracy of chromatographic assays.
The ability to accurately quantify therapeutic peptides in biological fluids requires a selective isolation process, a high-resolution chromatography system (LC), and a high-sensitivity detector (MS). As these therapeutic peptides imitate and/or replace the activity of endogenous peptides, it is desirable for the detection process to differentiate the endogenous and exogenous compounds. Although therapeutic peptides exhibit multiple charged states, the more specific high molecular weights typically observed for such peptides benefit from a mass spectrometer with an upper mass range in the region of 2000 Da on both quadrupoles, for the successful analysis of higher mass precursor or product ions.
In this application note, we describe the successful implementation of sample preparation and column chemistries using Waters’ ACQUITY UPLC H-Class System, and Xevo TQD Mass Spectrometer to develop an accurate, robust, and specific bioanalytical method for the quantification of exenatide in plasma.
|
System: |
ACQUITY UPLC H-Class including the High Temperature Column Heater |
|
Column: |
ACQUITY UPLC BEH300 C18 2.1 x 50 mm, 1.7 μm |
|
Column temp.: |
50 °C |
|
Solvent: |
0.2% formic acid in H2O (A), and 0.2% formic acid in acetonitrile (B) |
|
Gradient: |
20% A to 85% A/2 min |
|
Flow rate: |
200 μL/min |
|
Injection vol.: |
10 μL |
|
Run time: |
5 min |
|
MS Detector: |
Xevo TQD |
|
MRM data acquisition: |
838 → 948; |
|
|
838 → 396 |
|
Ion mode: |
ESI positive |
|
Capillary voltage: |
3.00 kV |
|
Desolvation gas flow: |
800 L/h |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
350 °C |
Exenatide was spiked into plasma, and extracted using an Oasis MAX µElution plate with 375 μL of plasma diluted 1:1 with 5% NH4OH. The samples were washed with 200 μL of 5% NH4OH, followed by 200 μL of 20% acetonitrile, and eluted with 2x 25 µL of 50:40:5:5 Acetronitrile/IPA/ H2O/Formic acid. The resulting samples were diluted with 50 μL of H2O containing the internal standard glucagon-like peptide.
A 10-μL aliquot of the sample was injected onto the column.
Data was recorded and processed using MassLynx with TargetLynx Application Manager.
Peptide therapeutics, unlike small molecules, exhibit less toxicity; however, they also exhibit high clearance and potency. Therefore, it is essential for the analytical technique of choice to have a fast, robust, reproducible, and sensitive method that would ensure determination and quantification of the peptide therapeutics and/or its metabolites in biological samples.
LC-MS/MS systems enable the user to accurately characterize the pharmacokinetics (PK) of such therapeutic peptides, especially for the low concentration of drugs after the Tmax.
The Xevo TQD Mass Spectrometer is equipped with the proven ZSpray ionization source, maximizing sensitivity and robustness by the unique use of dual orthogonal geometry to enable efficient transmission of ions into the analyzer while, simultaneously, providing robust removal of non-ionized materials (neutrals).
Of the two MRM transitions mentioned in the Experimental section of this application note, the transition 838 → 946 was monitored for optimal resolution and sensitivity. Exenatide eluted with a retention time of 3.28 min, as shown in Figure 2. As can be seen from this data, the peak produced by the chromatography system is very symmetrical and has a width at the base of 8 s. The peak sharpness and its symmetrical nature allow for efficient processing and peak integration with good resolution from any endogenous interference.
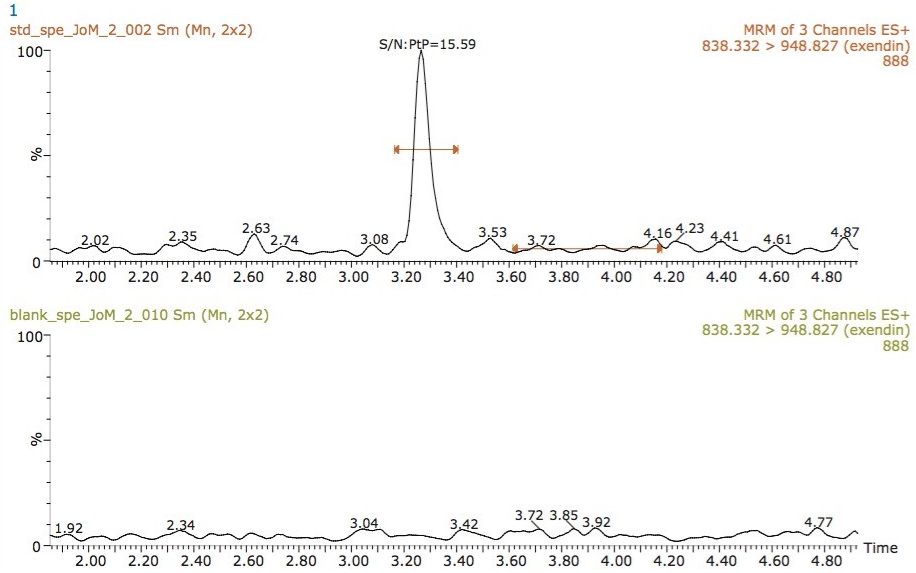
The limit of quantitation (LOQ) for the assay was determined to be 1 ng/mL of exenatide in plasma, with a signal to noise ratio of ~16:1. Figure 2 also illustrates the injection of an extracted plasma blank injection, immediately following the analysis of the 1000 ng/mL standard. This data shows that there is no discernable carryover in the blank chromatogram. The extremely low carryover exhibited by the ACQUITY UPLC H-Class System allows the full sensitivity of the Xevo TQD Mass Spectrometer to be exploited.
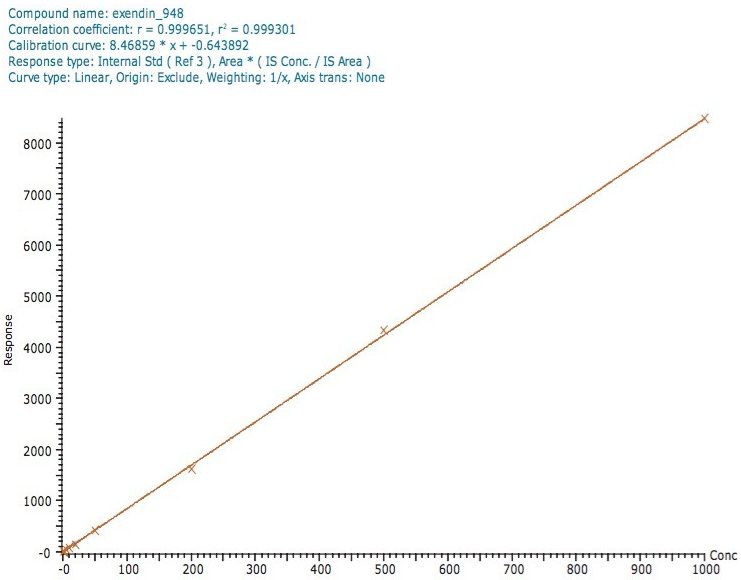
A calibration line obtained for the assay of exenatide is shown in Figure 3, with a correlation coefficient of 0.9993 using a 1/x weighting linear regression.
720004470, September 2012