
Chlorate and perchlorate have traditionally been analyzed by ion chromatography, which requires the use of specialized equipment. More recent methods involve LC-MS/MS, utilizing analytical columns highlighted in the Quick Polar Pesticides Methodology (QuPPe). In this technology brief, an alternative UPLC-MS/MS method is described with chromatographic separation achieved on a novel hydrophilic interaction liquid chromatography (HILIC) column, the Torus DEA.
The Torus DEA Column provides chromatographic characteristics that are well suited for retention and separation of anionic compounds such as chlorate and perchlorate.
Food can be contaminated by chlorate and perchlorate during different stages of production. Perchlorate can be present in food via the use of fertilizers, while chlorate can be present due to the use of chlorinated water during irrigation, crop washing, or disinfection of surfaces during food production. In 2008, chlorate was banned for use as a pesticide in the European Union (EU) and the maximum residue limit (MRL)1 for chlorate was set at 0.01 mg/kg (under revision).² There are currently no regulatory maximum limits for perchlorate in food within Europe.
The European Commission introduced reference levels for perchlorate³ (0.1 to 1.0 mg/kg depending on the commodity) to support trade, but they are evaluating the setting of maximum levels to replace current levels for intra-community trade. Chlorate and perchlorate have traditionally been analyzed by ion chromatography, which requires the use of specialized equipment.
More recent methods involve LC-MS/MS, utilizing analytical columns highlighted in the Quick Polar Pesticides Methodology (QuPPe).⁴ In this technology brief, an alternative UPLC-MS/MS method is described with chromatographic separation achieved on a novel hydrophilic interaction liquid chromatography (HILIC) column, the Torus DEA.
Waters Torus DEA column’s stationary phase consists of ethylene bridged hybrid (BEH) particles with tri-functionally bonded diethylamine ligands. The combination of the hydrophilic surface and the anion exchange properties of the ligands provide chromatographic characteristics that are well suited for retention and separation of anionic compounds such as chlorate and perchlorate. The use of BEH particles provides mechanical robustness to the column, allowing for the use of this stationary phase in small particle size column chemistries (sub-2-µm), thus facilitating the benefits of UltraPerformance Liquid Chromatography.
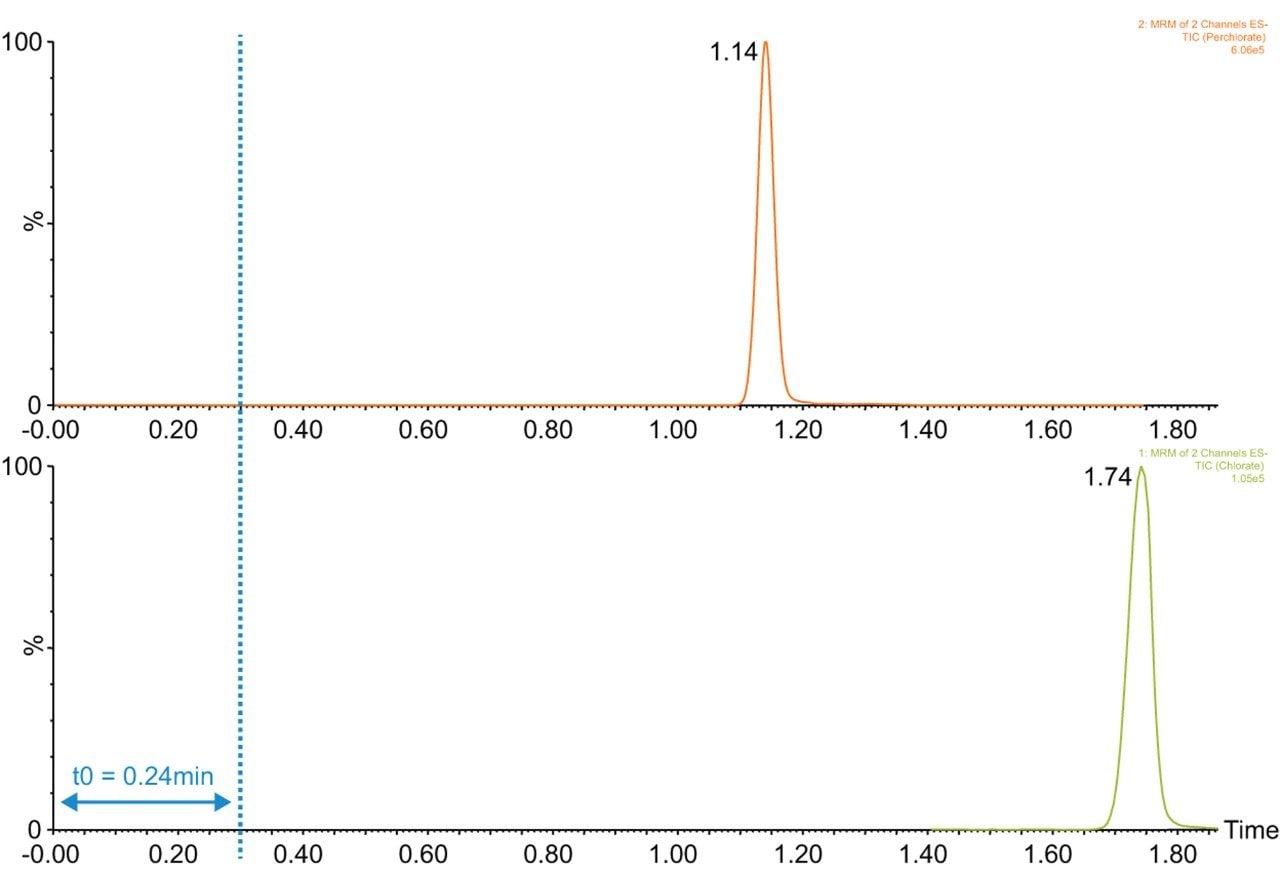
(UPLC) for this application, such as increased chromatographic resolution and shorter run times. An example chromatogram showing the retention and separation for the two compounds is shown in Figure 1, which also demonstrates the column void time (t0) for this method. The t0 is equivalent to 2 times the column void volume specified in the SANTE guidelines 11813/20175 due to the 0.5 mL/min flow rate used, alternative flow rates would result in a different t0. The total run time of the method including column re-equilibration is 7 minutes.
Samples of various representative food commodities were extracted using the QuPPe method and 5 µl of the filtered extracts were injected onto a 2.1 x 50 mm Torus DEA Column. The samples were analyzed using an ACQUITY UPLC I-Class System coupled with the Xevo TQ-XS, by applying an ammonium formate gradient to induce elution from the Torus DEA Column, 130 Å, 1.7 µm, 2.1 mm x 50 mm (p/n: 186007614).
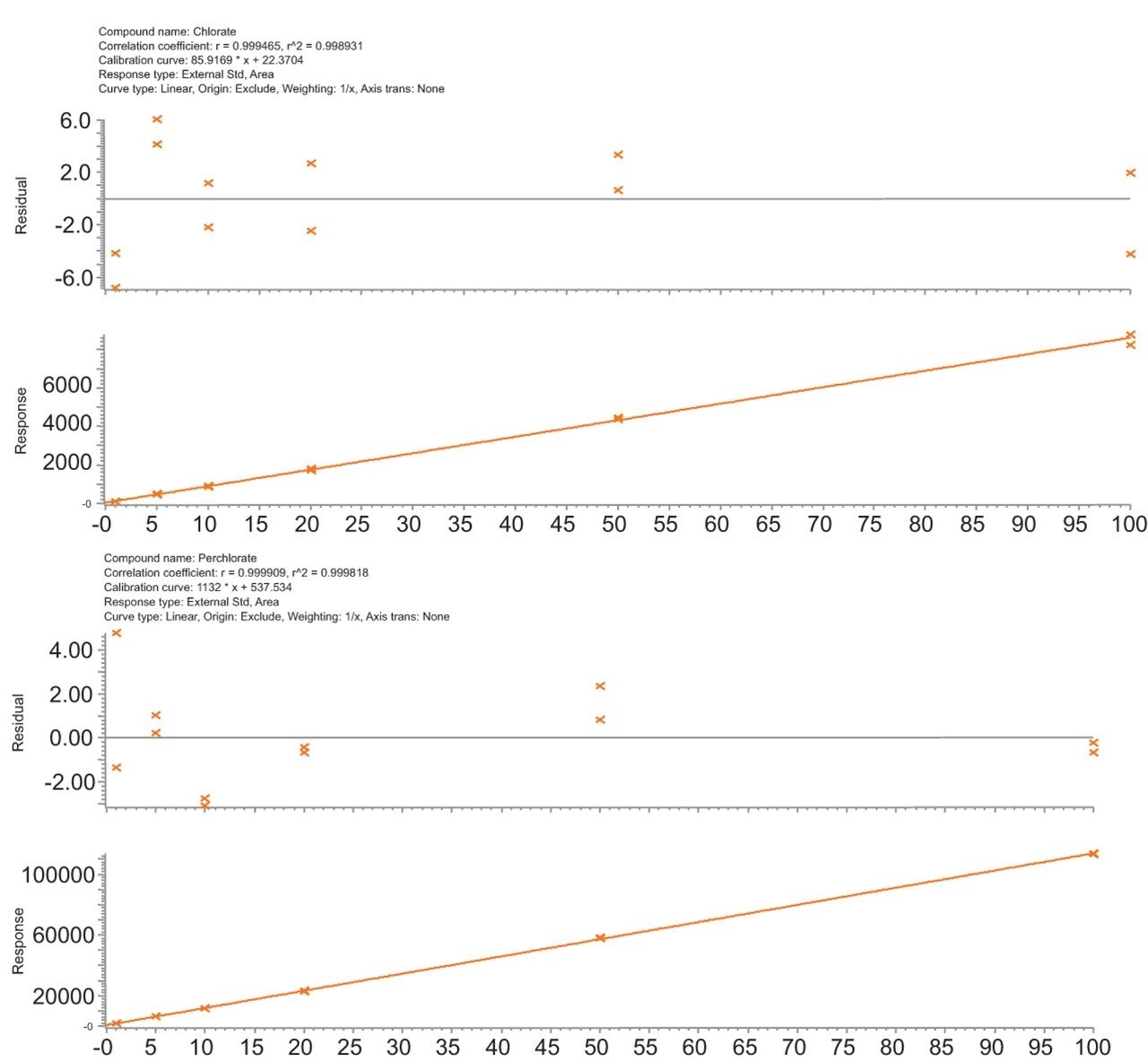
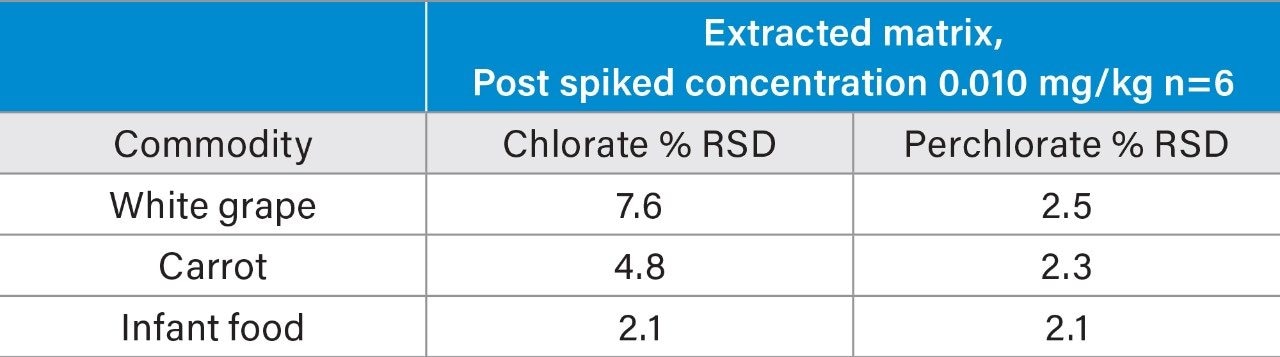
The LC-MS/MS method performance was assessed following the SANTE guidelines.⁵ Matrix-matched, bracketed calibration curves were run to test linearity. Example calibration curves in organic carrot are shown in Figure 2 for both compounds. Response was linear for both compounds over a range of 0.002 to 0.200 mg/kg (R²>0.995, residuals <20 %) in all tested matrices. Repeatability of the LC-MS/MS method was determined by injecting the 0.010 mg/kg level from the matrix matched curves (n=6). The repeatability (%RSD) from analysis of carrot, grape, and infant food can be seen in Figure 3.
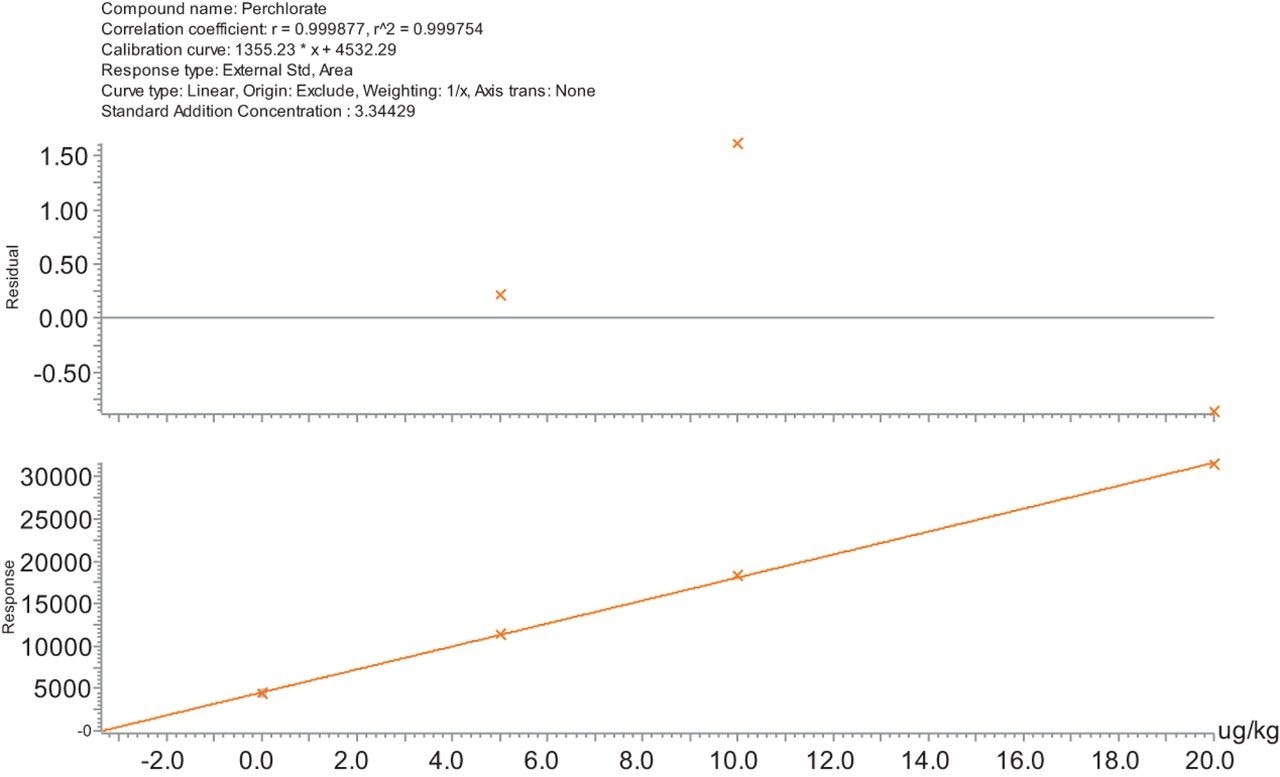
A possible residue of perchlorate was quantified in infant food, in the absence of an isotopically labelled internal standard, using a standard addition calibration within TargetLynx XS Application Manager. The retention time and ion ratio were within the tolerances defined in the SANTE guidelines. An example of this standard addition plot is shown in Figure 4, where the possible incurred residue was quantified at 0.003 mg/kg in infant food. All residuals were back calculated against the known added concentration automatically within TargetLynx and all were <6 %.
The Torus DEA Column provides excellent retention, retention time stability, and separation for the analysis of chlorate and perchlorate in the commodities tested when coupled to the ACQUITY UPLC I-Class and Xevo TQ-XS. Excellent linearity was obtained from the analysis of matrix-matched standards over the range 0.002 mg/kg to 0.200 mg/kg in the tested commodities. The measurements were shown to be repeatable from the replicate analyses of the matrix-matched standards prepared at 0.010 mg/kg in all three commodities (%RSD <8%) without the use of labelled standards. An incurred residue of perchlorate in infant food was quantified using standard addition in the absence of labelled standards.
720006421, November 2018