The use of biologic-based therapeutics, including monoclonal antibodies, has grown rapidly over the past twenty years. The complexity of these macromolecules requires the use of orthogonal analytical techniques for complete analysis and characterization. One technique, ion exchange chromatography (IEX), is used for analysis of charge heterogeneity biotherapeutics.
The use of biologic-based therapeutics, including monoclonal antibodies, has grown rapidly over the past twenty years. The complexity of these macromolecules requires the use of orthogonal analytical techniques for complete analysis and characterization. One technique, ion exchange chromatography (IEX), is used for analysis of charge heterogeneity biotherapeutics. Since each protein has a unique charge distribution, chromatographic selectivity can be adjusted by pH; however, these separations often lack resolution and consistency making method development more difficult. To address these issues, a solvent management system using pure solutions and concentrated stocks was developed. This system, capable of four solvent blending to prepare and adjust chromatographic mobile phases, can be combined with a high-resolution ion-exchange column to develop a separation for the lysine truncation variants of a chimeric monoclonal antibody. The weak-cation exchange column allows for method development over a range of pH’s and with multiple buffers to obtain the optimum separation of these charge variants. This will be illustrated with a specific method for a chimeric antibody.

A chimeric monoclonal antibody sample containing C-terminal lysine truncation variants was prepared at 1.25 mg/mL in 20 mM MES buffer, pH 6.
C-terminal lysine cleavage was performed using Carboxypeptidase B (CpB) (Worthington Biochemical Corp., p/n LS005304) prepared at 1 mg/mL. The monoclonal antibody (1000 µL, 1.25 mg/mL) and CpB (12.2 µL, 1 mg/mL) were combined. At predetermined time intervals (t= 0, 1, 2.5, 5, 7.5, 10, 12.5, 15, and 20 min), a 100 µL aliquot of the mixture was removed and combined with glacial acetic acid (1.7 µL) to halt the reaction.
|
LC system: |
ACQUITY UPLC H-Class Bio System with Auto•Blend Plus Technology |
|
Detector: |
PDA Detection with Titanium Flow Cell |
|
Wavelength: |
280 nm |
|
Sampling rate: |
20 pts/sec |
|
Filter time constant: |
Normal |
|
Column: |
Protein-Pak Hi Res IEX CM, 4.6 x 100 mm, 7 μm (P/N 186004929) |
|
Column temp.: |
30 °C |
|
Sample temp.: |
4 °C |
|
Injection volume: |
10 μL |
|
Flow rate: |
0.5 mL/min |
|
Mobile phase A: |
100 mM Sodium Phosphate, monobasic, or 100 mM MES monohydrate |
|
Mobile phase B: |
100 mM Sodium Phosphate, dibasic, or 100 mM MES sodium salt |
|
Mobile phase C: |
1000 mM Sodium Chloride (NaCl) |
|
Mobile phase D: |
Water |
|
Purge and wash solvents: |
20mM Sodium Phosphate, pH 6.0 or 20mM MES, pH 6.0 |
|
Gradient: |
0-10% C in 60 min, (pH specified in figures) |
|
Software: |
Empower 2 with Auto•Blend Plus Technology |
Charge heterogeneity of a monoclonal antibody may be caused by several structural changes including C-terminal lysine processing.1 When present in biopharmaceuticals, these charge variants are often monitored throughout manufacturing to ensure control of the process. In the following study, an IEX method was developed to confirm and quantify the presence of C-terminal lysine truncation variants in a chimeric monoclonal antibody therapeutic. Method development was performed on a weak-cation exchange column (Protein-Pak Hi Res CM, 4.6 x 100 mm, 7 µm) by manipulating pH and ionic strength. A four solution blending system was used to make pH buffer adjustments by using a weak acid (line A) and the cognate base (line B). Sodium chloride (NaCl, line C) and water (line D) were used to adjust the ionic strength of the buffer. These adjustments were performed using Auto•Blend Plus Technology, which allowed the gradient to be expressed directly in terms of pH and ionic strength. The operating software automatically calculates the percentage of acid and base required for the specified pH from the known or measured pKa of the selected buffer system.
Two common cation-exc hange buffers, sodium phosphate and MES ((N-Morpholino) ethanesulfonic acid), were compared. For each buffer system, the effect of pH was studied (Figures 1 and 2). A single salt gradient (0-100 mM NaCl in 60 minutes) was tested over a pH range of 6.0-7.0.
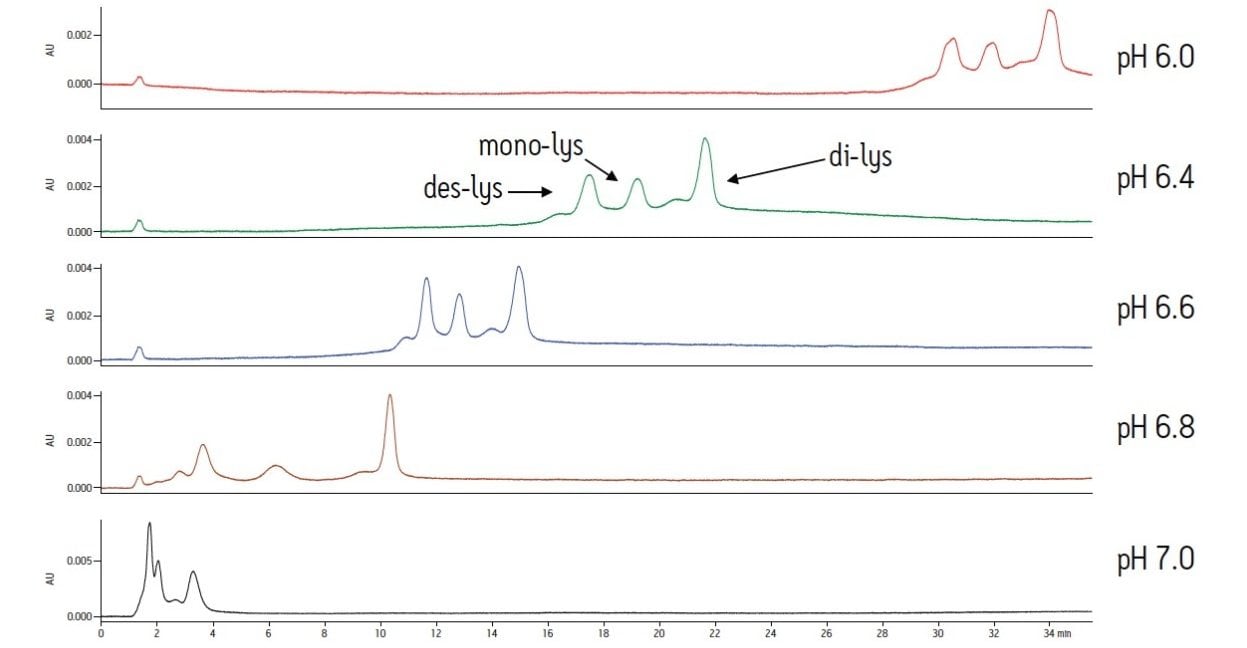
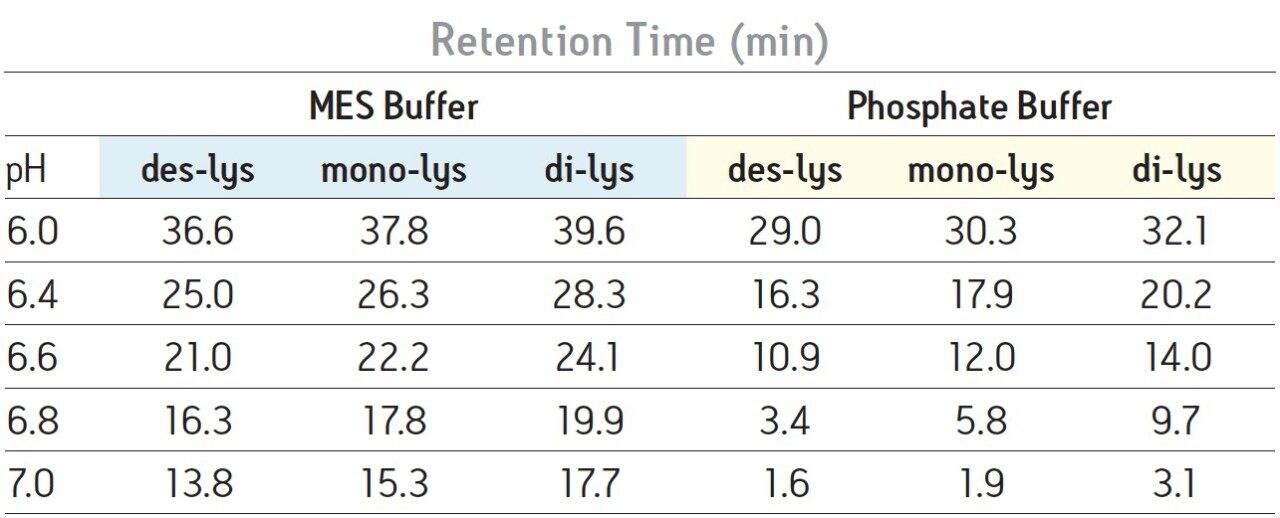
With a sodium phosphate buffer system, the resolution of the monoclonal antibody (des-lys) and the C-terminal lysine truncation variants varies with pH. Higher pH corresponds to earlier elution of the chimeric antibody and the lysine truncation vari-ants. This behavior is typical of cation-exchange chromatography since the overall positive charge of a protein decreases as pH increases, thereby resulting in elution of the analytes at a lower ionic strength. The non-truncation species (des-lys) elutes at retention times ranging from 3.4 minutes at pH 6.8 to 29.0 minutes at pH 6.0 (Table 1). This corresponds to NaCl concentrations ranging from 4 mM at pH 6.8 to 68 mM at pH 6.0 (Table 2). A similar trend is observed for the antibody with a single C-terminal lysine truncation (mono-lys). However, under the same conditions, the doubly C-terminal lysine truncation variant (di-lys) exhibits a smaller shift in both retention time and NaCl concentration. This difference in retentivity of the variants as pH is increased yields higher resolution between the mono-lys and di-lys variants at pH 6.8 (Figure 1) as compared to lower pH. A mobile phase pH of 7 results in almost no retention for the antibody and the charged variants.
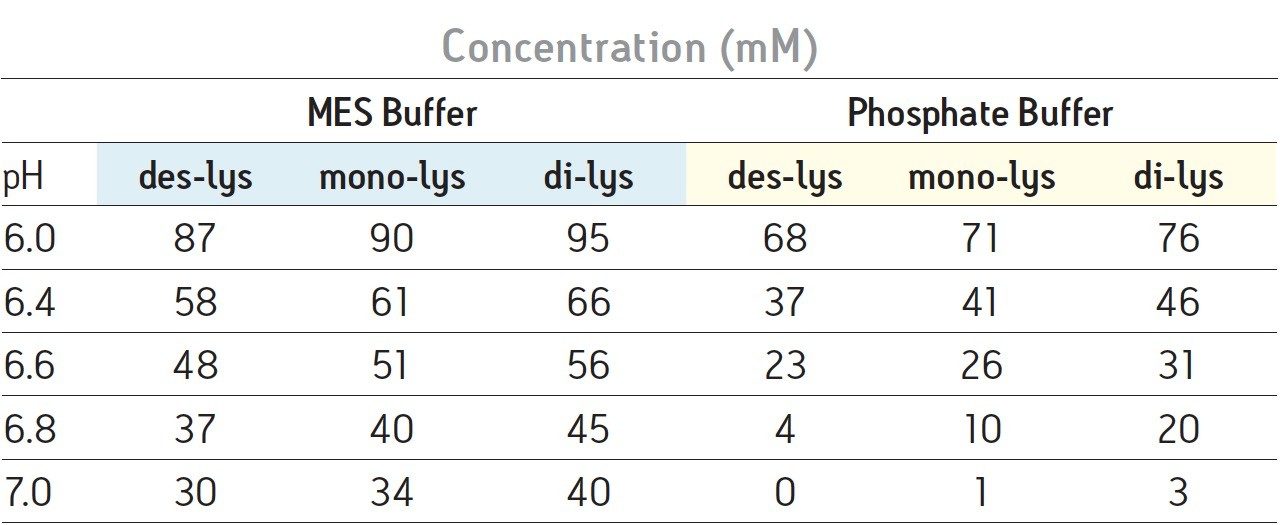
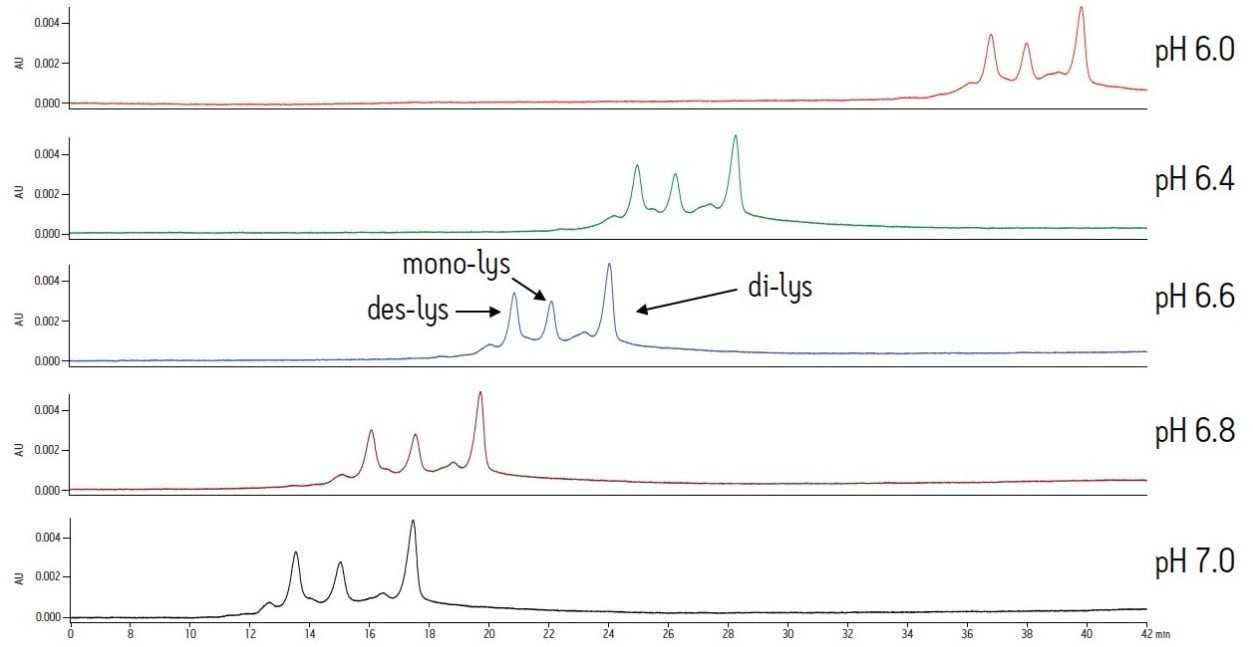
The same study with an MES buffer system demonstrates the effect of buffer composition on an IEX separation. As similar to the sodium phosphate buffer system, a higher pH results in lower retention for the antibody and the charge variants. However, resolution is not significantly affected (Figure 2). Overall, the MES buffer results in later elution of the analytes when compared to the sodium phosphate buffer system. Varying pH with the MES buffer produces changes in retention. Selectivity is not significantly altered.
Differences in the retention observed between the two buffers can be attributed to the different ionic strengths of each buffer system. The ionic strength of a buffer is based on the total number of ions contributed by both the sodium chloride and the buffering agent. For the two buffering agents used in this screening, this difference is largely due to the different number of sodium ions present. The sodium phosphate buffer system combines the mono- and di-basic forms of phosphate. Therefore, when the weak acid and cognate base are in equal proportion, three sodium ions are contributed by the buffer. In contrast, the MES buffer system is comprised of the weak acid form and the cognate sodium base. When both acid and base are in equal proportion in the MES buffer system, an amount of sodium equimolar to the cognate base (10 mM) is contributed by the buffer. Thus, when the ionic strength of NaCl is held constant and both buffering agents are at the same concentration and pH, the phosphate buffer system will have a greater ionic strength when compared to the MES buffer due to the additional sodium ions present. In quantitative terms, at a pH of 6.0 the sodium phosphate buffer system contributes an additional 22.7 mM of sodium ions to the mobile phase while the MES buffer system adds an additional 8.9 mM of sodium ions. This difference in contributing ions results in earlier elution of the antibody and the C-terminal lysine truncation variants with a sodium phosphate buffer (Table 1). As pH is increased, sodium phosphate contributes even more sodium ions in the form of the base (32.3 mM at pH 7) as compared to MES buffer system (17.8 mM sodium ions), resulting in a greater ionic strength at a constant NaCl concentration, and thus a greater retention time shift with pH as compared to MES buffer system (Table 1).
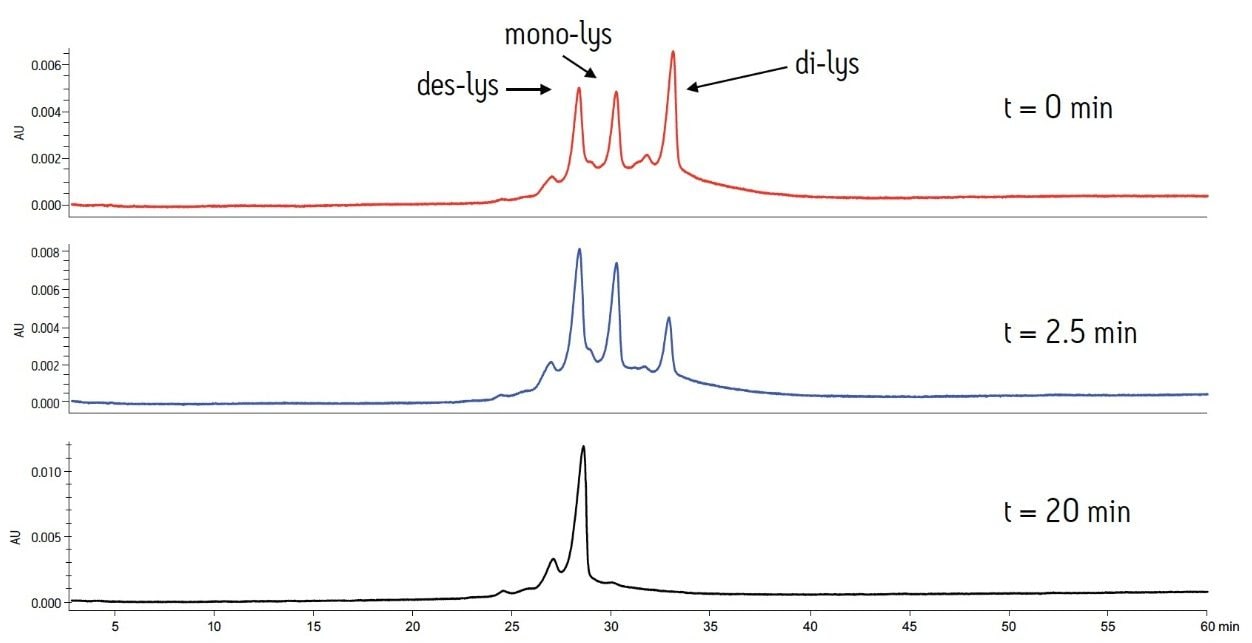
The separation method was used to confirm the identity of the peaks. To confirm the C-terminal lysine truncation variants, the antibody biotherapeutic was treated with carboxypeptidase B following previously published protocols.2,3 The separation was performed with MES buffer system at a pH of 6.6. These buffer conditions allow for separation of the antibody and the C-terminal lysine truncation variants in addition to the analysis of smaller eluting acidic or basic variants. The reaction was monitored over a period of 20 minutes. At predetermined time points, an aliquot of the sample was removed and combined with acetic acid to halt the reaction.
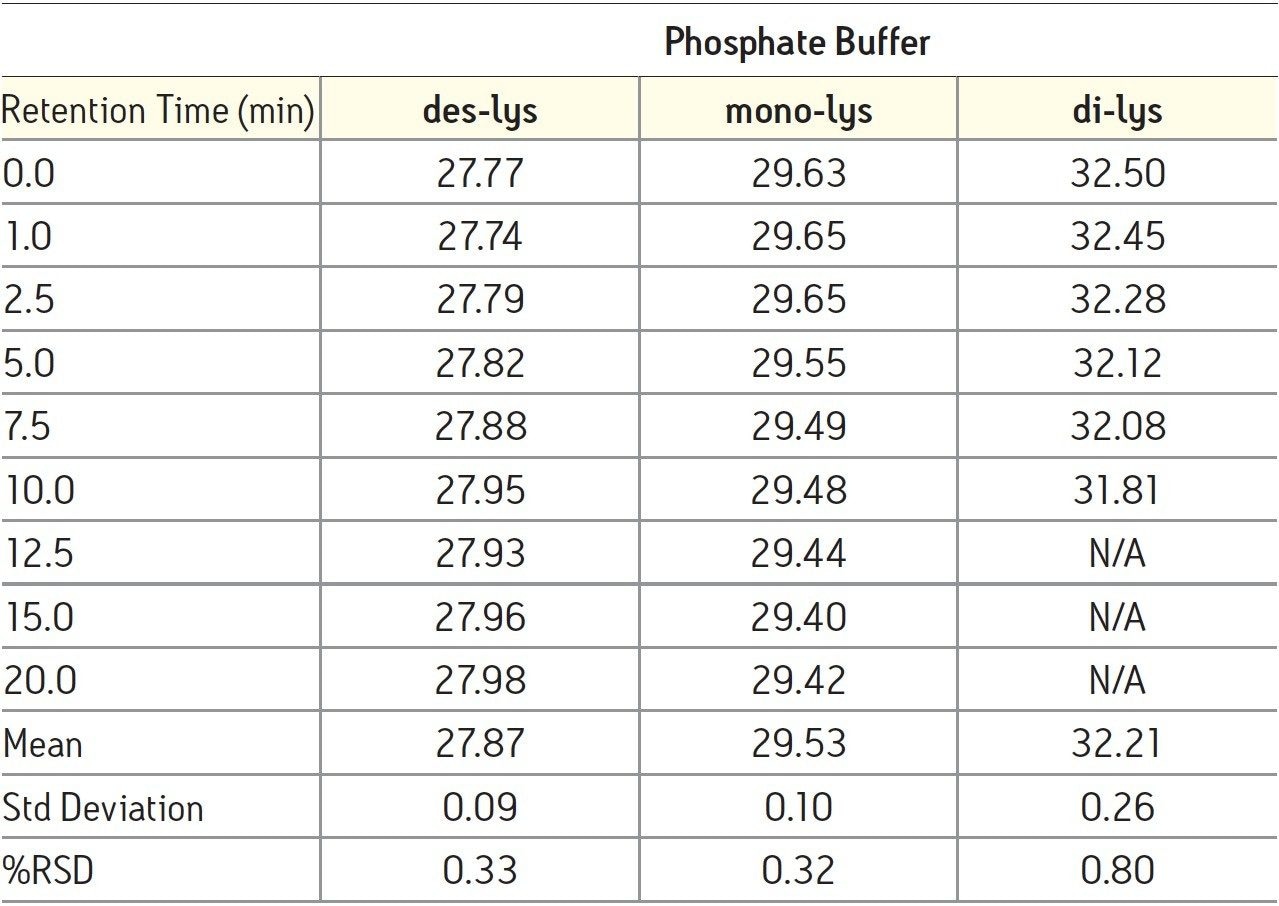
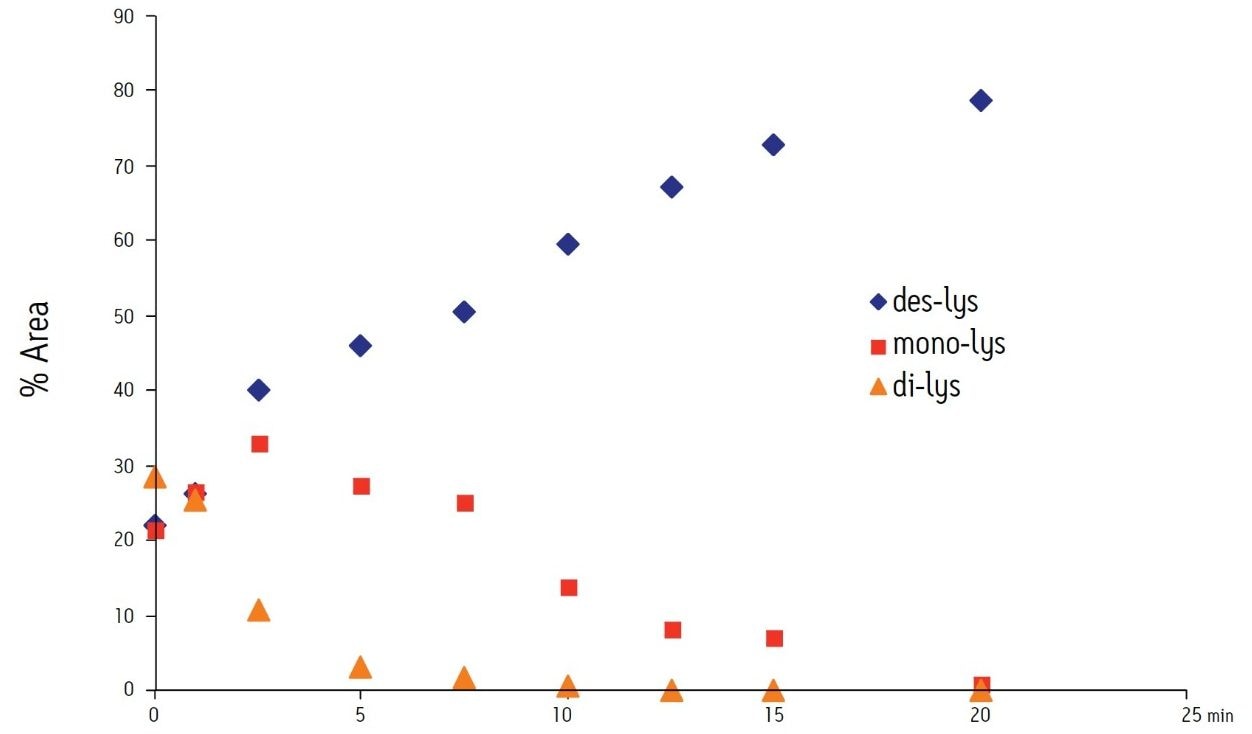
The time course study of the reaction demonstrates both the reproducibility of the IEX method and the conversion of the di-lys and mono-lys forms to the des-lys form. The retention time reproducibility was less than 0.1% RSD for all of the major components over the time course study (Table 3). The conversion of the mono-lys and di-lys variants to the des-lys antibody is also confirmed by analysis of % peak area. Within the first 2.5 minutes of the reaction, the latest eluting peak (di-lys) shows the greatest decrease in % peak area (Figure 3). In that same time period, the des-lys and mono-lys both show an increase in % peak area (Figures 3 and 4). Subsequent time point analyses show a continual increase in % peak area for des-lys, while both mono-lys and di-lys variants continue to exhibit a decrease in % peak area and are almost undetectable at 20 minutes (Figure 3). These results are consistent with conversion of the di-lys and mono-lys variants to the des-lys antibody.
The heterogeneity of a biopharmaceutical monoclonal antibody from C-terminal lysine truncation is typically monitored throughout manufacturing to ensure process stability and insure quality control. For these charge variants, the Protein-Pak Hi Res CM column provides a tool for the analysis and confirmation of a chimeric antibody and its C-terminal lysine truncation variants. T he column, in combination with the ACQUITY H-Class Bio System and Auto•Blend Plus Technology, allows for simplified pH screening and evaluation of multiple buffer systems. Method development studies for the chimeric antibody demonstrate the dramatic affect of pH with a sodium phosphate buffer, which is partially due to the ionic strength of the buffering agent. In contrast, minimal resolution effects are observed with varying pH in a MES buffer system. All of the screening studies performed are simplified with the use of a four-solvent blending system and Auto•Blend Plus Technology. The resulting separation provides a robust method for analysis and confirmation of a monoclonal antibody and its C-terminal lysine truncation variants.
Auto•Blend Plus Tutorial, IEX Technical Brief, Literature Reference 720003601en.
720003836, March 2011