The BEH300 C4 column chemistry was designed specifically so that the high resolution characteristic of UPLC would be available for protein analyses. Using the BEH300 C4 column chemistry, quantitation of a commercially-available monoclonal antibody is shown in this application note, demonstrating the linear range. Also shown is an example for detection and quantitation of trace-level impurities.
The quantitative analysis of biopharmaceutical proteins is required at various stages in the development process. Depending on the phase and specific focus of a biotherapeutic lifecycle, quantitation can vary from an estimation of the target protein (+/- 10%) to quantitation of impurities at levels < 1% of the target protein. To accommodate these varying requirements, it is not only necessary to determine the best range for the assay, but to assure that the required dynamic range can be readily and reliably achieved. The BEH300 C4 column chemistry was designed specifically so that the high resolution characteristic of UPLC would be available for protein analyses. Using the BEH300 C4 column chemistry, quantitation of a commercially-available monoclonal antibody is shown in this application note, demonstrating the linear range. Also shown is an example for detection and quantitation of trace-level impurities.
Linearity Samples: Fully humanized IgG4 prepared in 0.1% CF3COOH, for mass load targets of 0.05, 0.1, 0.5, 1, 5, 7.5, 10, 20, 30, 40, 50 μg on column (3.3 μL injection).
Trace impurity quantitation samples: 0.1%, 0.2% 0.5%, 1%, 2.5%, and 5% murine IgG1 in the presence of a constant 50 μg Humanized IgG4 on column (3 µL injection).
|
LC system: |
ACQUITY UPLC system (fitted with the Peptide needle and 425 μL peptide mixer) |
|
Detector: |
ACQUITY TUV detector |
|
Sanple vial: |
Waters Certified Total Recovery vials (P/N 186000384c) |
|
Column: |
ACQUITY UPLC BEH300 C4, 2.1 x 50 mm, 1.7 μm (P/N 186004495) |
|
Column temp: |
80 °C |
|
Sample temp: |
10 °C |
|
Injection volume: |
3 μL or 3.3 μL |
|
Injection mode: |
Partial loop |
|
Flow rate: |
0.2 mL/min |
|
Mobile Phase A: |
0.1% CF3COOH in water |
|
Mobile Phase B: |
0.1% CF3COOH in IPA |
|
Weak needle wash: |
0.1% CF3COOH in 5% acetonitrile |
|
Strong needle wash: |
0.1% CF3COOH in 75% acetonitrile |
|
Seal wash: |
50/50 acetonitrile/water |
|
Gradient: |
20 – 37% B in 14.7 min; 1 min regeneration at 90%; 13 min reequilibration at 20% B UV detection at 220 and 280 nm |
|
Software: |
Empower 2 CDS |
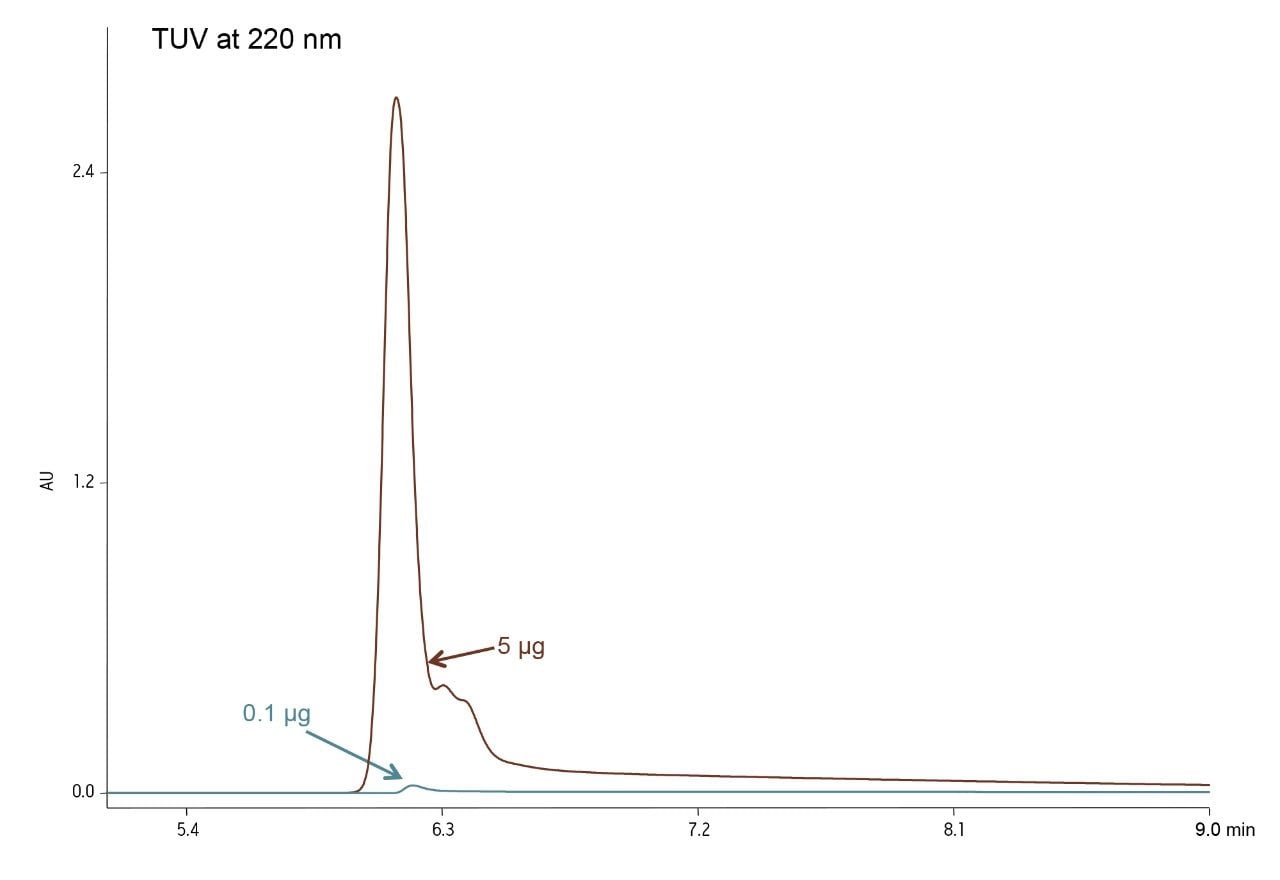
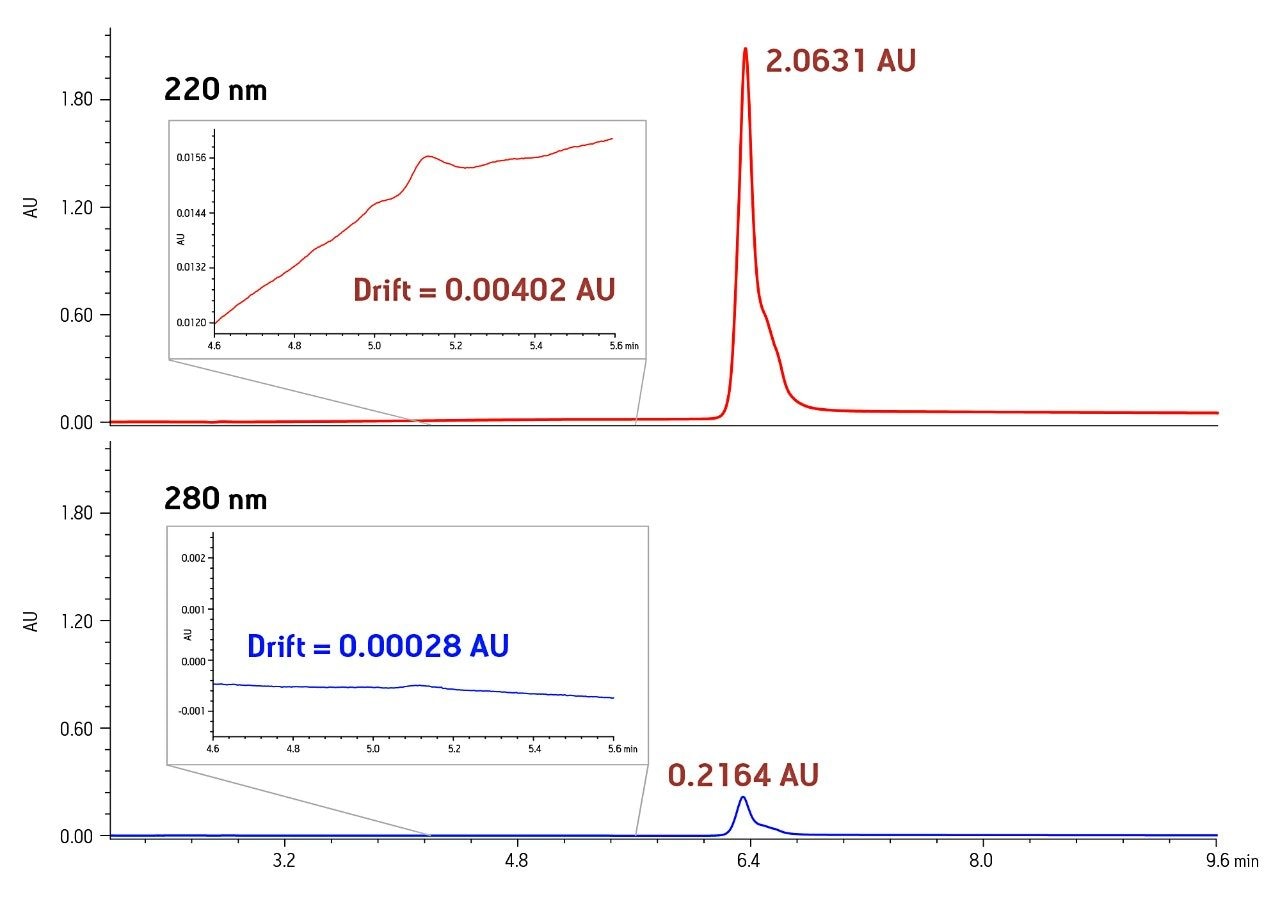
UV detection at low wavelengths (e.g. 220 nm) is often used for protein analysis because of its high sensitivity. At low wavelength, low levels of a protein can be detected. However, detector saturation limits the dynamic range to a 50-fold range of concentrations (Figure 1). Saturation of the detector at 220 nm occurs between 5 and 7.5 µg of protein injected on a 2.1 x 50 mm column The analysis of the same mass load and injection volume of humanized IgG4 showed almost a 10-fold difference in peak height between the chromatograms at 220 nm and 280 nm (Figure 2). The reduced peak height at 280 nm was also accompanied by changes in baseline characteristics. The low end of the linear range, that is the lower limit of quantitation, is dependent upon both drift and short term detector noise. The noise is similar at the two wavelengths, on the order of 0.00002 AU. The drift, however, is more than ten-fold greater at 220 nm. Such anomalies directly affect the accurate integration of peaks. The increased saturation limit and reduced baseline anomalies combine to give a wider usable dynamic range at 280 nm.
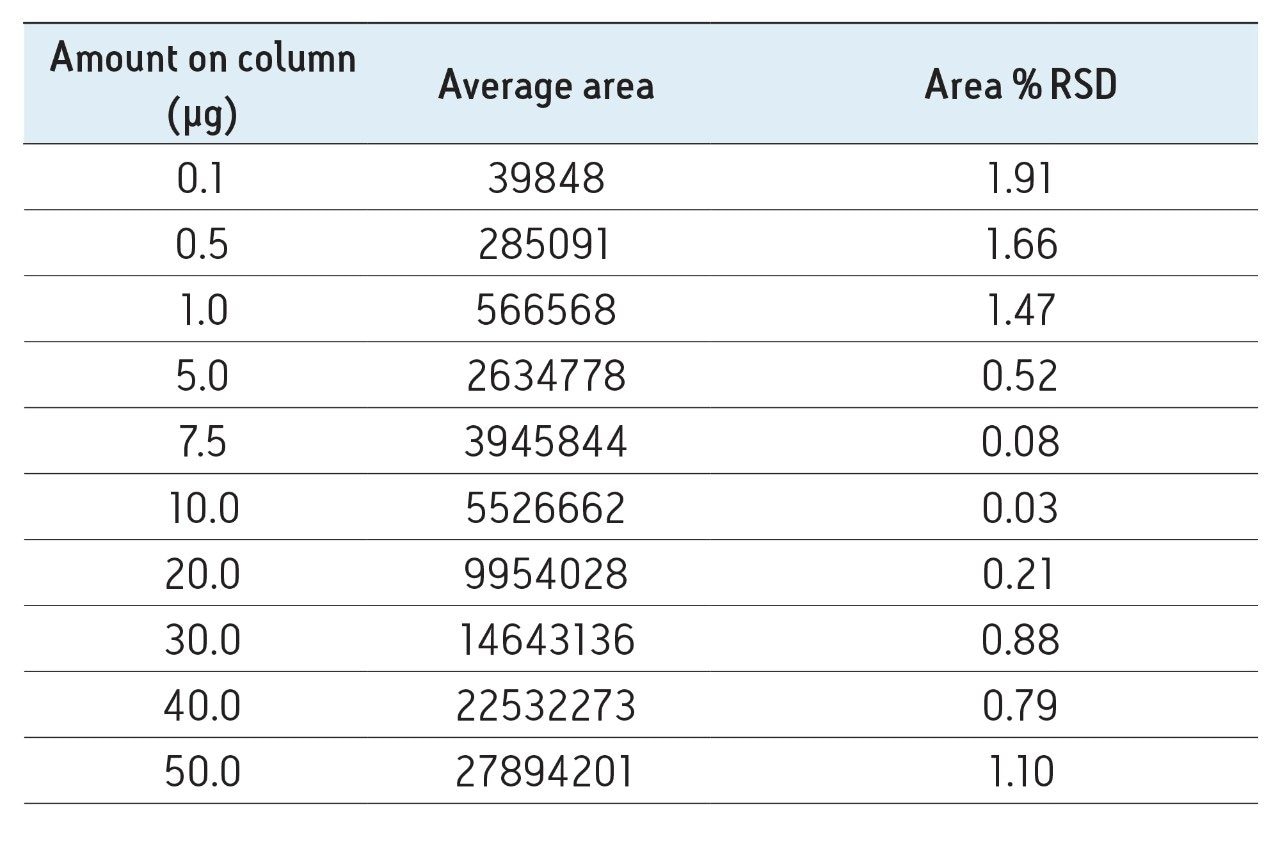
The humanized IgG4 was used to determine the linear range with UV detection at 280 nm. Ten different mass loads (0.1 – 50 µg) of protein were analyzed in triplicate at a constant injection volume of 3 µL. At 280 nm, the highest protein load of 50 µg on-column was below the detector saturation limit, and the same variant forms of the protein were still detected. The method was linear across all levels tested, with an R2 value of 0.994. The variability of multiple injections was also less that 2% across the full set (Table 1).
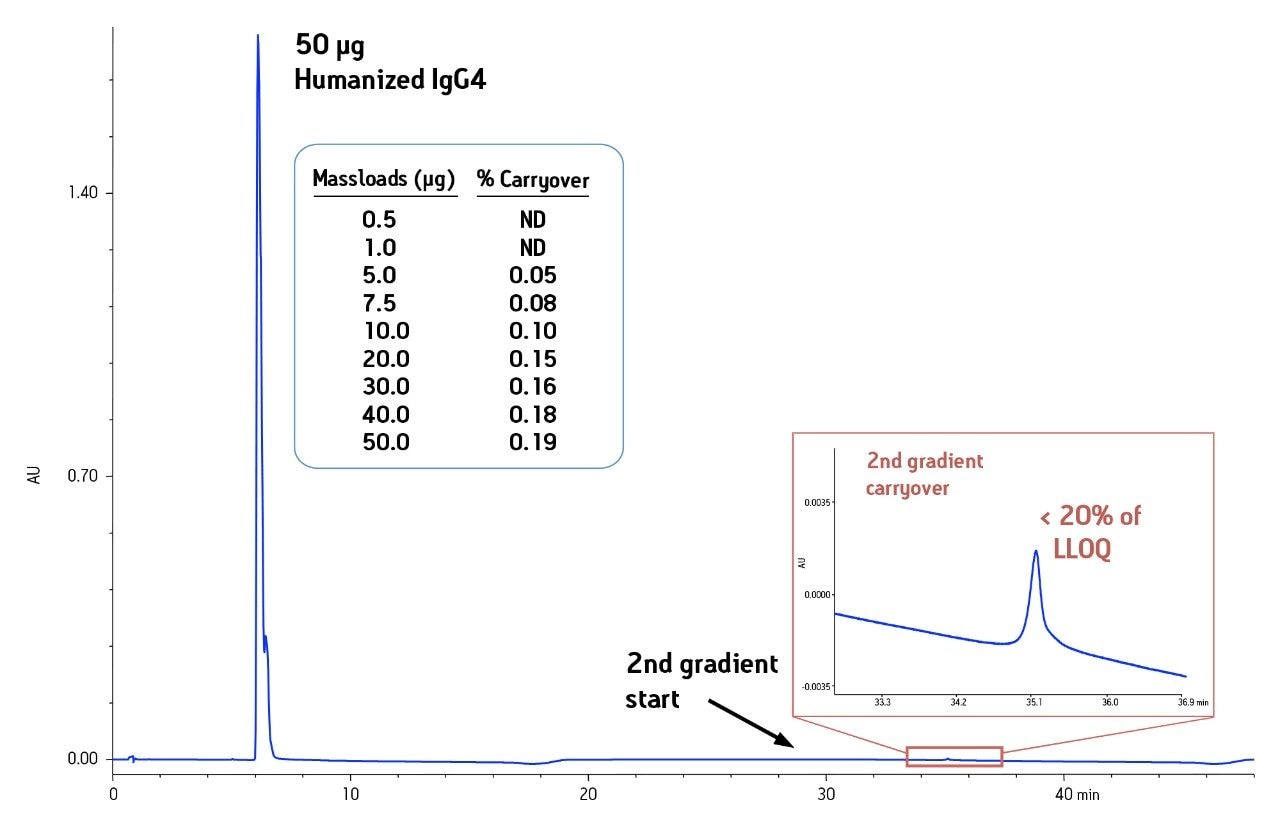
Validation of any quantitative assay must demonstrate the absence of carryover. Observation of carryover has been particularly problematic in protein assays. In developing an application, two sources of carryover are recognized, the fluid path of the instrument and the chromatographic column. Column-related carryover, or memory effect, can be measured by running a second gradient immediately following the sample analysis without making another injection. This internal gradient would show memory effects as a peak at the corresponding retention time in the second gradient.1 This procedure was used for the same set of samples shown in Table 1 (Figure 3). All levels tested showed < 0.2% carryover for the column, which was less than 20% of the lower limit of quantitation. The ability to quantitate proteins from 0.1 – 50 µg with minimal carryover makes this a good method for accurate and consistent quantitation of low level impurities.
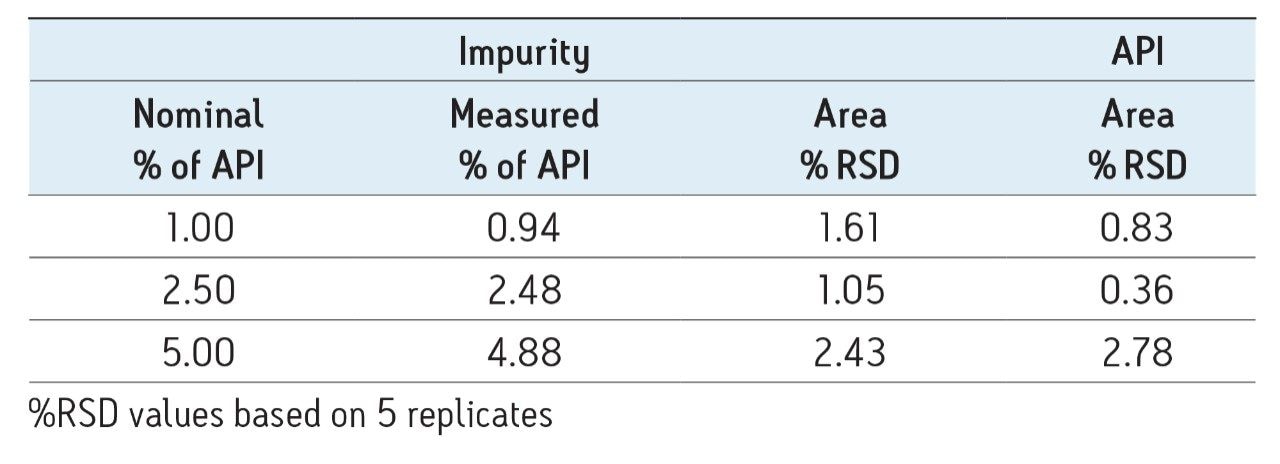
The above experiments show the utility of quantitating a single protein peak using reversed-phase chromatography. At various stages in the biopharmaceutical development process, it is necessary to quantitate individual proteins in a mixture, most often proteins that are low abundance impurities in the presence of a larger amount of another protein. To demonstrate this, a series of spiked IgG samples was analyzed. These samples contained 50 μg of a humanized IgG with spiked levels of a murine IgG at levels of 1 – 5% of the larger IgG protein peak. As shown in Table 2, the measured impurity amount matched the expected values with good reproducibility.
The sensitivity and resolution offered by reversed-phase chromatography make it a good investigative tool for the characterization of proteins. Consistent analyses are observed over a wide range of protein concentrations without any distortion of the chromatographic retention or peak shape. Reliable and reproducible quantitative results are readily obtained for monoclonal antibodies using the BEH300 C4 column chemistry. Minimal protein carryover on this column chemistry helps to improve the accuracy of the quantitative results. Through the combination of ACQUITY UPLC and the BEH300 C4 column chemistry, fast and reliable quantitative results are obtained at any point in the lifecycle of a protein biotherapeutic.
720003944, April 2011