
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the selectivity advantages of the ACQUITY UPC2 stationary phases and factors that contribute to a streamlined workflow, including significant savings in analysis and sample prep time, solvent costs, and solvent waste disposal.
Steroid biosynthesis is a complex metabolic pathway utilizing simple precursors to synthesize multiple steroidal forms. This biosynthetic pathway is unique to animals and provides a common target for antibiotics and other anti-infective drugs. Precise and accurate steroid analysis is critical for the development of steroid-based therapeutics. Typical analysis methods utilize GC-MS, which require sample derivatization and lengthy analysis times (~25 minutes), or LC-MS with typical analysis times of four to 12 minutes. Many of the steroid structures are closely related making their analysis challenging even when using the selectivity of mass spectrometric detection. Chromatographic separation is, therefore, essential for analysis of steroids and steroid derivatives resulting in long analysis times. Convergence chromatography, with CO2 as the primary mobile phase, presents a unique opportunity to provide rapid and precise analyses for these structurally related compounds, as shown in Figure 1.
A mixture of nine steroids was prepared at a concentration of 0.2 mg/mL each, using a diluent of 88:12 methanol/ethanol. Steroids used included the following: androstenedione, estrone, 17α-hydroxyprogesterone, testosterone, 11-deoxycortisol, estradiol, corticosterone, aldosterone, and cortisol.
All data was collected on an ACQUITY UltraPerformance Convergence Chromatography (UPC2) System with photodiode array (PDA) detection. The steroid sample was screened on three different ACQUITY UPC2 column chemistries including: BEH, BEH 2-EP, and CSH Fluoro-Phenyl, using a 1.7 μm particle size in a 3.0 x 50.0 mm column dimension. The mobile phases were CO2 with methanol as a co-solvent. A two-minute screening gradient was used from 2% to 17% methanol at a flow rate of 3.65 mL/min, and a temperature of 40 °C. The Automatic Back Pressure Regulator (ABPR) was set to 1800 psi. Data was collected at 220 nm (compensated for 380 to 480 nm). The injection volume was 1 μL.
The chromatograms shown in Figure 2 demonstrate the selectivity differences of the ACQUITY UPC2 stationary phases, as well as the inherent speed of this chromatographic technique, with a significant reduction in analysis times compared to alternative techniques.
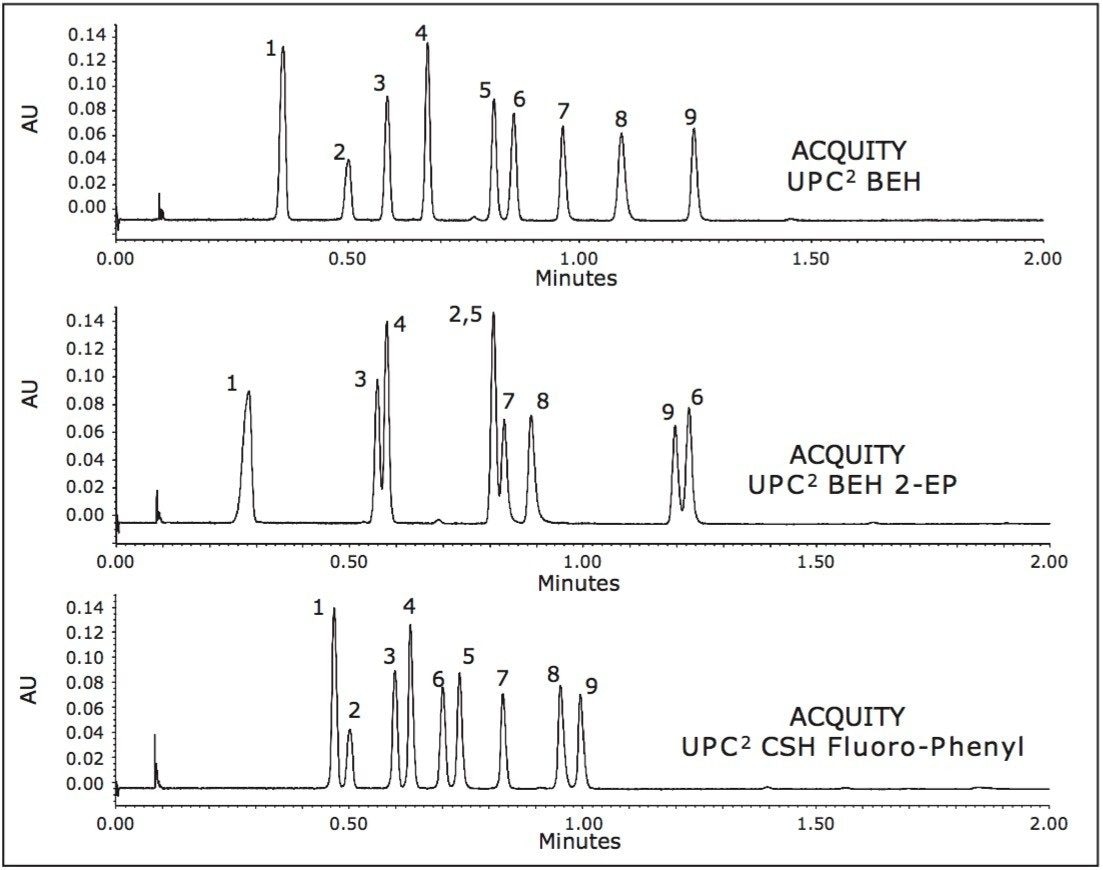
Without the need for derivitization (required for GC analysis), samples can be analyzed directly in organic extraction solvents, omitting the need for diluent exchange for compatibility with reversed-phase LC methods. These factors combined yield a streamlined workflow with significant savings in analysis and sample prep time, solvent costs, and solvent waste disposal.
720004527, December 2012