
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates that the Waters Fraction Manager – Analytical (WFM-A) provides an analytical scale solution for the isolation and enrichment of therapeutic oligonucleotides from process-related impurities that can impact product safety and efficacy.
The Waters Fraction Manager (WFM-A) is used to efficiently isolate and enrich therapeutic oligonucleotides from process-related impurities.
The production of oligonucleotides with high yields via automated stepwise synthetic methods is well established. This process, while very efficient with yields greater than 99% per coupling step, can still result in total yields that are lower than desirable as oligonucleotide length increases. Even for an oligonucleotide of modest sequence length (21 mer), a coupling efficiency of 99% would generate a product with a maximum purity of 81% without further purification.1 Characterization of synthetic products must be carried out prior to use to ensure product identity and purity.2 This is of particular importance in therapeutic applications such as siRNA gene silencing where by-products of synthesis such as failed sequences and production impurities can result in off-target gene silencing and reduced drug potency.1
In practice, commercial synthesis of oligonucleotides produces yields with sufficient purity for many applications; however, when production purity is not sufficient, purification strategies that are efficient and readily implemented are desirable.
The Waters Fraction Manager – Analytical (WFM-A) System is a fraction collector for UPLC systems (Figure 1) that minimizes fraction loss and carryover for efficient collection of small amounts of material with high purity and recovery.3 The WFM-A is designed for straightforward integration into an existing UPLC instrument stack and can be controlled using the Waters Empower Chromatography Data Software as shown in Figure 2. The instrument can be programmed to collect fractions in an automated fashion using time, slope, or threshold. In this example, the Collection Event Table was programmed using a processed chromatogram of a commercially synthesized 21-mer siRNA to isolate the parent siRNA from its impurities (Figure 2, green box).

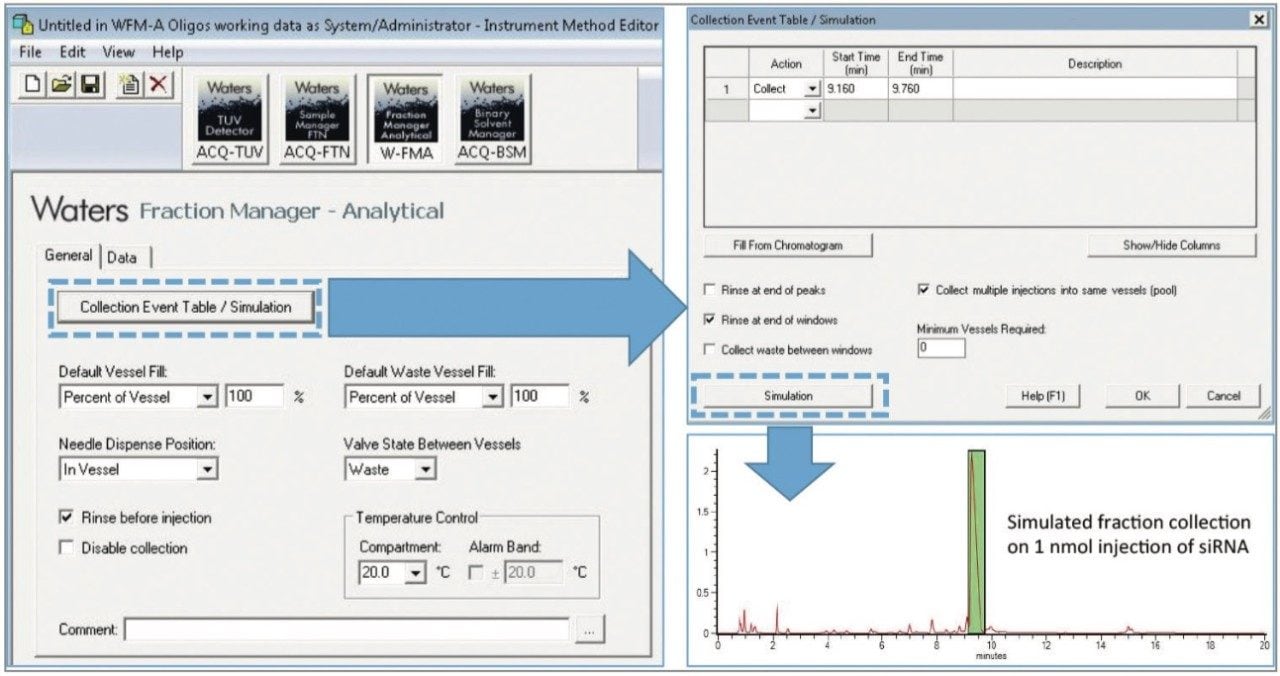
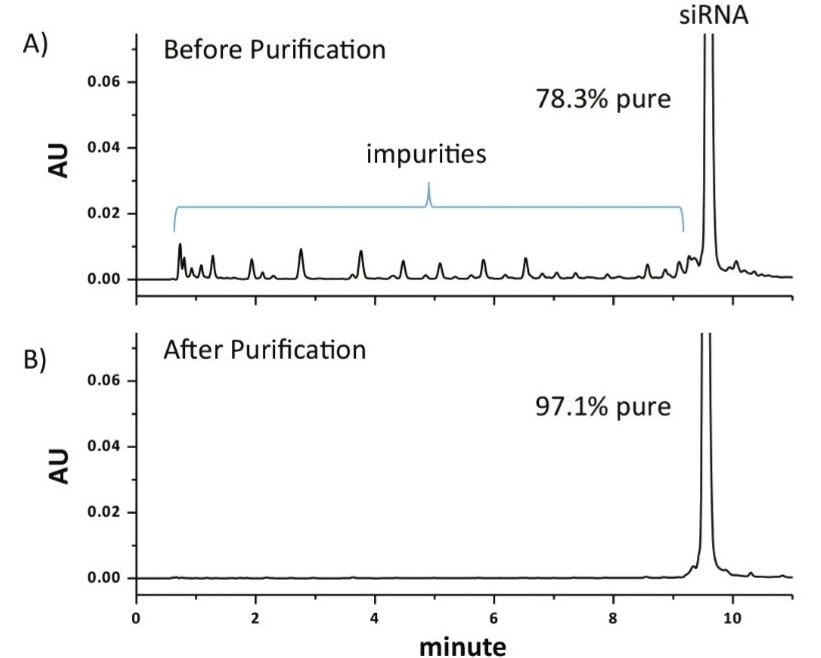
After isolating the parent siRNA using a collection window from 9.16 – 9.76 min for two consecutive injections of 1 nmol of synthesized product, the collected fractions were dried down via vacuum centrifuge and re-constituted at the same concentration as the initial starting material to facilitate comparison. As shown in Figure 3, under equivalent mass load conditions for the parent peak, the purity of the commercially synthesized siRNA was increased to more than 97% purity using the Waters Fraction Manager, which is more suitable for therapeutic applications such as gene silencing experiments.1
The Waters Fraction Manager – Analytical (WFM-A) provides an analytical scale solution for the isolation and enrichment of therapeutic oligonucleotides from process-related impurities that can impact drug product safety and efficacy. The compact design of the WFM-A readily integrates with Waters low dispersion UPLC systems facilitating small scale workflows that offer high recovery with minimal carry-over. The WFM-A is a fraction collection solution that promotes efficient workflows that are ideal for analytical scale purification, enrichment, and impurity isolation applications.
720005923, February 2017