An Ultra Performance Liquid Chromatography Mass Spectrometry Method for the Analysis of Non-derivatized Gentamicin Components
Abstract
Gentamicin, an aminoglycoside that lacks chromophores, is commonly used as a broad-spectrum antibiotic for gram-negative and some gram-positive bacterial infections. It consists of different related components differing in methylation and toxicity. As such, the analysis and quantification of each component is particularly important for the safety of patients and to comply with regulatory agencies. In this study a Hydrophilic Interaction Liquid Chromatography (HILIC) based method for fast separation of gentamicin C1, C1a, C2, C2a for ACQUITY™ Ultra Performance Liquid Chromatography™ H-Class System, using an Atlantis™ Premier BEH™ Z-HILIC Column, with an ACQUITY QDa™ Mass Spectroscopy Detector was developed. The method was tested on an ophthalmic gentamicin drug formulation and variation in packing material for the Z-HILIC chemistry was investigated. The method requires no derivatization or fluorinated pairing agents, unlike many of the current methods found in literature. Separation uses a ten minute isocratic elution which reduces the re-equilibration time between samples, providing a rapid accurate and reproducible separation of gentamicin components.
Benefits
- Utilizes a simple to use compact ACQUITY QDa Detector
- No derivatization or fluorinated pairing agents needed
- Isocratic separation allowing reduced equilibration time, thus a faster analysis of samples
- Same instrument and similar method can be used to separate many aminoglycosides (AMGs)
Introduction
Gentamicin, a broad-spectrum aminoglycoside antibiotic, is a mixture of four main components gentamicin C1, C1a, C2, C2a, and one minor component C2b, structures can be found in Figure 1. Gentamicin is created through fermentation of Micromonospora purpurea. This fermentation creates a circumstance where different manufacturers may have different compositions of these compounds based on the strain selected for the biosynthesis. The different “C” compounds of gentamicin only differ slightly in their methylation, but they have different levels of toxicity (notably ototoxicity and nephrotoxicity). This coupled with the drugs narrow therapeutic index make it important to be able to separate the constituent compounds.1 The importance becomes more evident when the several deaths in 1998 caused by gentamicin administration are taken into consideration; although not fully proven, it is hypothesized that the deaths were caused by related compounds sisomycin and precursor histamine.2
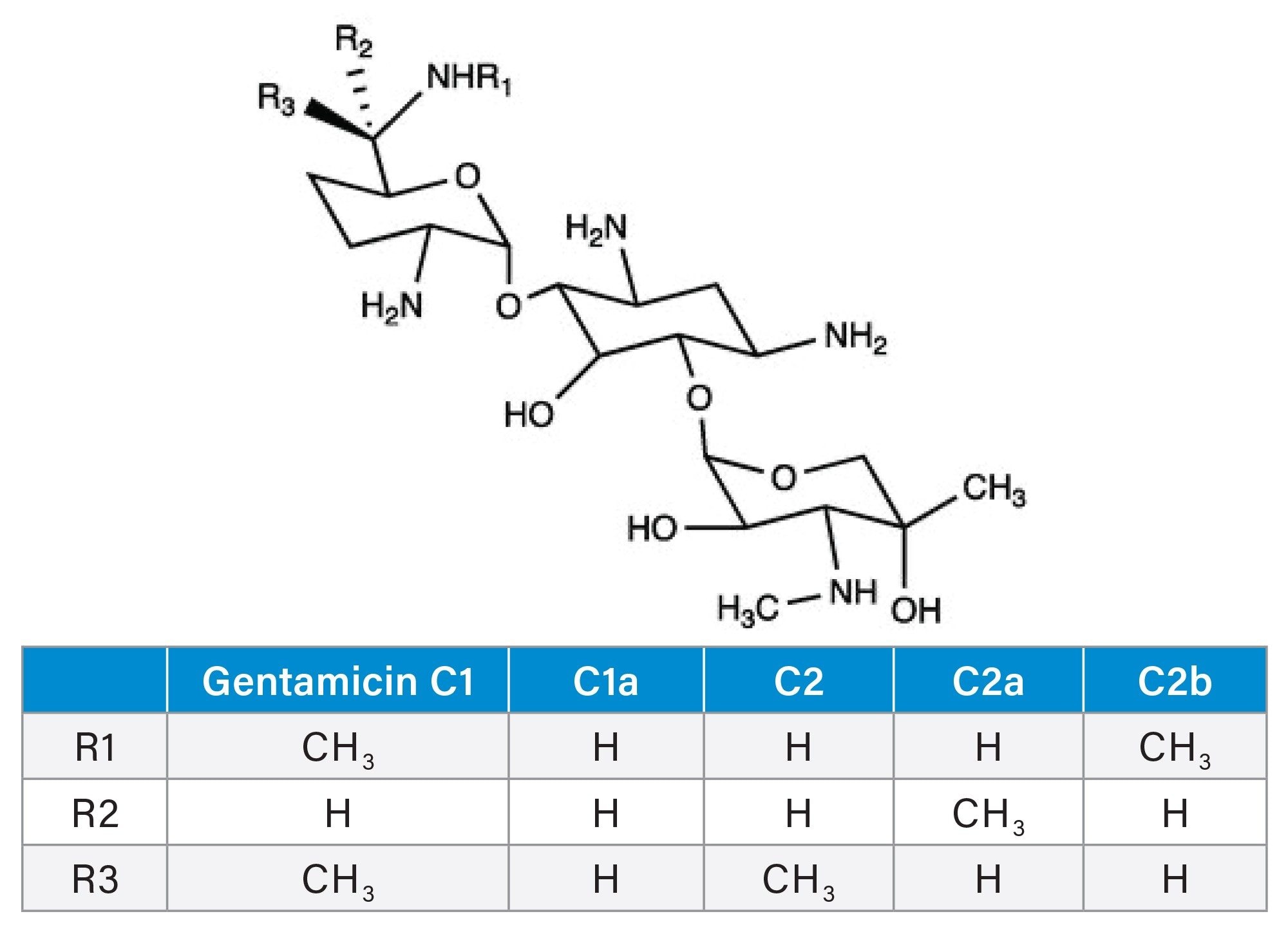
This method uses an Atlantis Premier BEH Z-HILIC Column, a column packed with a zwitterionic (sulfobetaine) stationary phase and utilizing MaxPeak™ HPS Technology; run in HILIC mode. This zwitterionic stationary phase allows for good retention of highly polar chemicals and thus good separation of similar compounds like what is found in gentamicin mixtures.3 Several methods using C18 and HILIC columns have been developed that use fluorinated pairing agents or derivatization to aid in separating the compounds.4–6 These methods tend to take a long time and require high ionic strengths.6 Other researchers from East China University have found success with different chemistries such as zwitterionic click TE-Cys columns. Their method takes longer and requires higher ionic strength mobile phases as well as an evaporative light scattering detector, which can become problematic with buildup of ionic compounds.6
The method outlined in this application note was designed to separate gentamicin into its four main components, all while avoiding fluorinated pairing agents and derivatization. This is beneficial as when fluorinated pairing agents are used with mass spectroscopy, they can cause ion suppression and contamination of the instrument.3 Avoiding derivatization is also helpful as it reduces the sample preparation time and makes the method simpler overall. Another advantage of the method developed in this paper is that a similar method had been developed by Waters that can separate and quantitate a panel of AMGs using the same instrument configuration, only differing in ammonium formate concentration and method parameters.7 The following method uses the Atlantis Premier BEH Z-HILIC Column in combination with the AQCUITY QDa Mass Detector for the separation and the quantification of gentamicin components.
Experimental
Columns for Reproducibility
Three Atlantis Premier BEH Z-HILIC 1.7 μm 2.1 x 100 mm Columns were obtained, each column packed with different batches (batch # 101,102,104), also a fourth column containing batch 101 was packed in-house using stainless steel 2.1 x 100 mm column hardware.
Sample Description
Two samples of Gentamicin sulfate were obtained through Sigma Aldrich (G1914, PHR0177). Stocks were prepared in polypropylene (PP) with LCMS grade water (Burdick & Jackson) and stored in PP containers and stored in a freezer. Sample of 100 μg/mL of gentamicin sulfate were made and stored at 10 ˚C.
Gentamicin Drug Formulation Samples
Samples were obtained through Asaman located in Avon Massachusetts, 0.3% gentamicin ophthalmic eyedrop solutions of 5 mL manufactured by Bausch and Lomb, and Greenstone/Pacific Pharma were used. Samples were diluted to 100 μg/mL using MS grade water.
Method Conditions
Method uses a 250 μL mixer changed from 100 μL stock mixer. (p/n: 205000719).
LC Conditions
|
LC system: |
ACQUITY Ultra Performance LC H-Class Plus (250 µL Mixer) |
|
Detection: |
ACQUITY QDa Mass Spec (performance) |
|
Vials: |
Poly Propylene 2 mL |
|
Column(s): |
Atlantis Premier BEH Z-HILIC Column 1.7 μm 2.1 x 100 mm |
|
Column temperature: |
55 °C |
|
Sample temperature: |
5 °C |
|
Injection volume: |
3 μL |
|
Flow rate: |
0.8 mL/min |
|
Mobile phase A: |
80 mmol Ammonium Formate in MS grade water Burdick & Jackson (non-adjusted pH: ~6.3) |
|
Mobile phase B: |
0.1% (v/v) Formic acid in ACN MS grade Burdick & Jackson |
|
Mobile phase percentage: |
A:48% B:52% |
MS Conditions
|
MS system: |
ACQUITY QDa |
|
Ionization mode: |
Positive |
|
Acquisition range: |
300 to 630 Da |
|
Capillary voltage: |
0.8 kV |
|
Probe temperature: |
600 °C |
|
Cone voltage: |
12 V |
Data Management
|
Chromatography software: |
Empower™ 3 |
Results and Discussion
Separation of gentamicin had been noted during another project while working with a panel of aminoglycosides.7 The gentamicin peak had been separated into two clear peaks. Although the two peaks were not fully resolved, the conditions of these separations were used as a starting point for a one factor at a time method development approach.
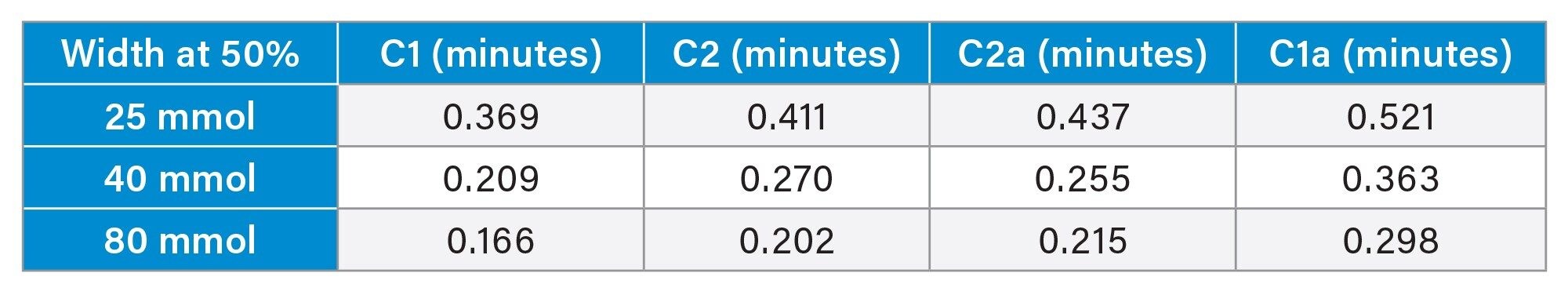
The effect of the mobile phase composition was pursued first. Initially a gradient method was investigated starting below 50% aqueous and ending at 85%. A very gradual gradient created the best separations when it comes to gradient methods, so isocratic elution was investigated. Isocratic elutions from 85% down to 40% aqueous were checked. It was found that an aqueous percentage of 48% resulted in very retained peaks with good shape. Lower aqueous percentages were tested but were found to decrease the sharpness of peaks resulting in further difficulty with integration. Since the peaks are highly retained, the column temperature was increased from 50 °C to 55 °C to reduce retention time while maintaining peak shape. The next variable that was evaluated was the buffer concentration. Ammonium formate of 8, 25, 40, and 80 mM were tested. Results show that the ammonium formate concentration has a large impact on the separations. As can be seen in Table 1, the higher concentration resulted in narrower peaks and increased sharpness. The higher ammonium formate concentration was also found to decrease run times while maintaining or improving the separation as can be seen in Figure 2. As literature suggested, the higher concentration the better, although higher concentrations can negatively impact the QDa performance by increasing baseline noise and causing other sensitivity issues. Generally, ammonium formate concentration is kept around 10 mmol when used with QDA but 80 mmol works consistently in this method.8 The consistency with 80 mmol is potentially due to increasing the mixer size from a stock 100 μL to 250 μL mixer. Other sources state that a lower pH, around four, is optimal2–4 with this method an unadjusted pH (about 6.3) was found to be better giving sharper peaks with higher responses and more consistent results. Higher MS Probe temp of 600 °C was found to give better sensitivity.
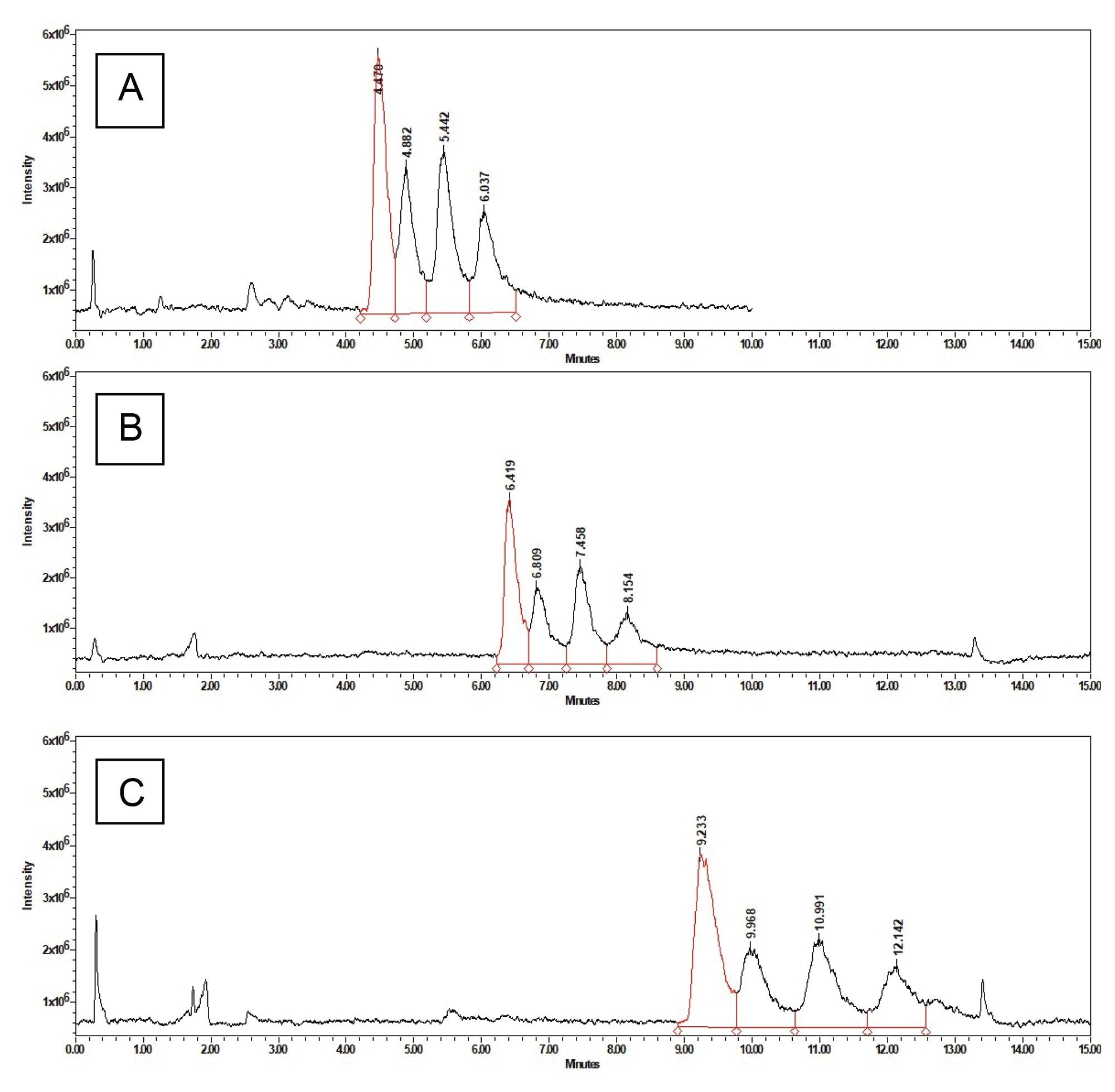
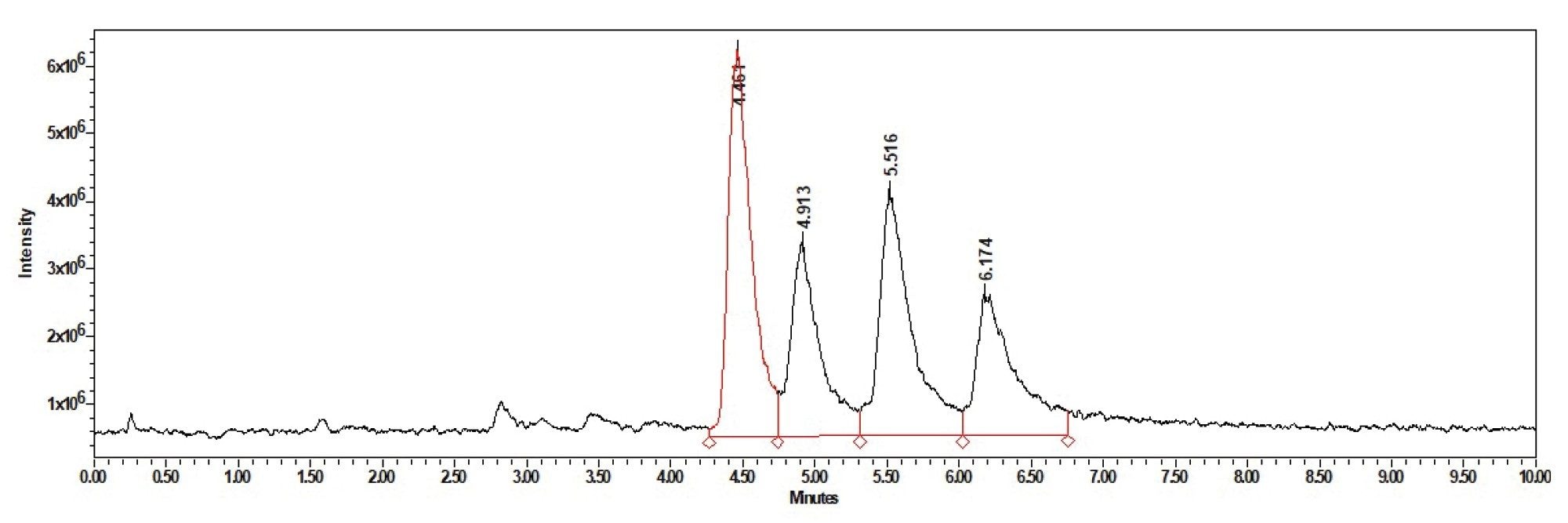
The method developed was able to successfully separate the 4 main components of the Gentamicin mixture C1, C1a, C2, and C2a. The resolution of each peak was greater than 1.390 for all injections and some were found to have resolutions of up to 1.873 as seen in Figure 3.
The processing method and more specifically the integration parameters appear to be the biggest point of deviation, especially the determination of the end of the C1a peak. Table 4 shows the average retention time and the standard deviation across six different injections. As the relative standard deviation is below 1% it can be concluded that the method is reproducible. Accounting for the difficulty with the integration of the last peak the method still maintains a relative standard deviation of the integrated area of less than 5%. These exact values can be found in Table 2 and Table 3.
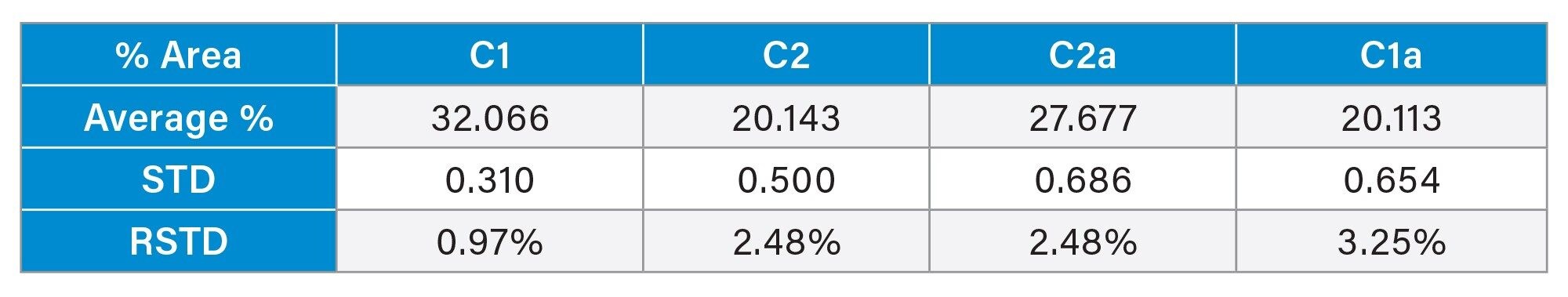
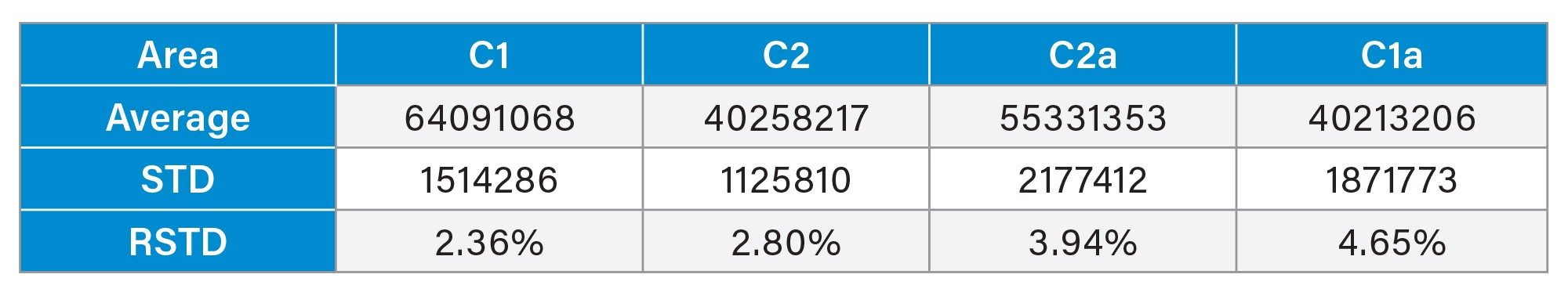
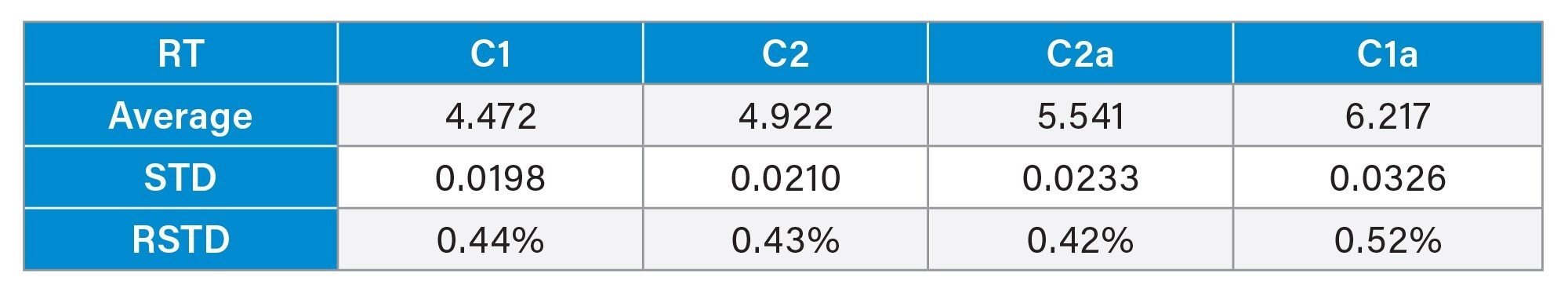
Note that equilibration of the column from a different mobile phase composition can take up to thirty minutes, otherwise retention time can be inconsistent. SIR was considered for analysis of the compounds but due to the high ionic strength many ammonium formate adducts were formed creating a variety of masses making SIR response low. TIC was used to avoid the low response seen in SIR. The compounds are also very similar so cross talk is also a problem to consider and made separation of the compounds necessary with a single quadrupole mass spectrometer method.
Column Chemistry Batch Reproducibility
Column to column reproducibility was investigated to ensure the method is compatible across different batches of packing material. Reproducibility was tested for the method developed in this paper and the previous study done on aminoglycosides. The effect of MaxPeak HPS technology was also checked versus a bare metal stainless steel column, no substantial difference was seen in the peak area. HPS increased retention time with an aminoglycoside panel. Relative standard deviation of gentamicin samples slightly decreased with the HPS as seen in Table 5. The reproducibility was found to be acceptable with both methods. The retention times and peak shape would change a small amount from different batches of packing material. Each column had less then a 10% relative standard deviation in peak area across six different injections. The retention times on each column were below 1% RSTD and retention times across all columns and all injections were less than 5%. Thus, the method is reproducible with normal variation in column chemistry.

Formulations of gentamicin eyedrops were obtained from Greenstone/Pacific Pharma and Bausch & Lomb. The eyedrops were a 3 mg/mL concentration making dilution necessary to not overload the detector. Samples were diluted with MS grade water to a final concentration of 100 μg/mL. Bausch & Lomb contained 0.01% benzalkonium chloride, a preservative, and Greenstone contained an unknown concentration. Each manufacturer has a different mixture of inactive ingredients both containing a saline phosphate buffer solution, pH adjusted to 6.5–7.5. Greenstone had extra additives consisting of edetate disodium and polyvinyl alcohol.
Although the ingredients in the eyedrops differ, the method was able to separate the gentamicin into its constituent compounds. The benzalkonium was not retained on the column and came out close to the void volume time eluting at 0.245 and 0.244 minutes for the Greenstone sample and the Bausch & Lomb sample respectively. The splitting pattern confirms the identity of the benzalkonium peak. Greenstone does not report the concentration of the benzalkonium chloride, but the peak area was more then double that of Bausch & Lomb suggesting a difference in concentration. All four gentamicin peaks were resolved as seen in Figure 4 and Figure 5.
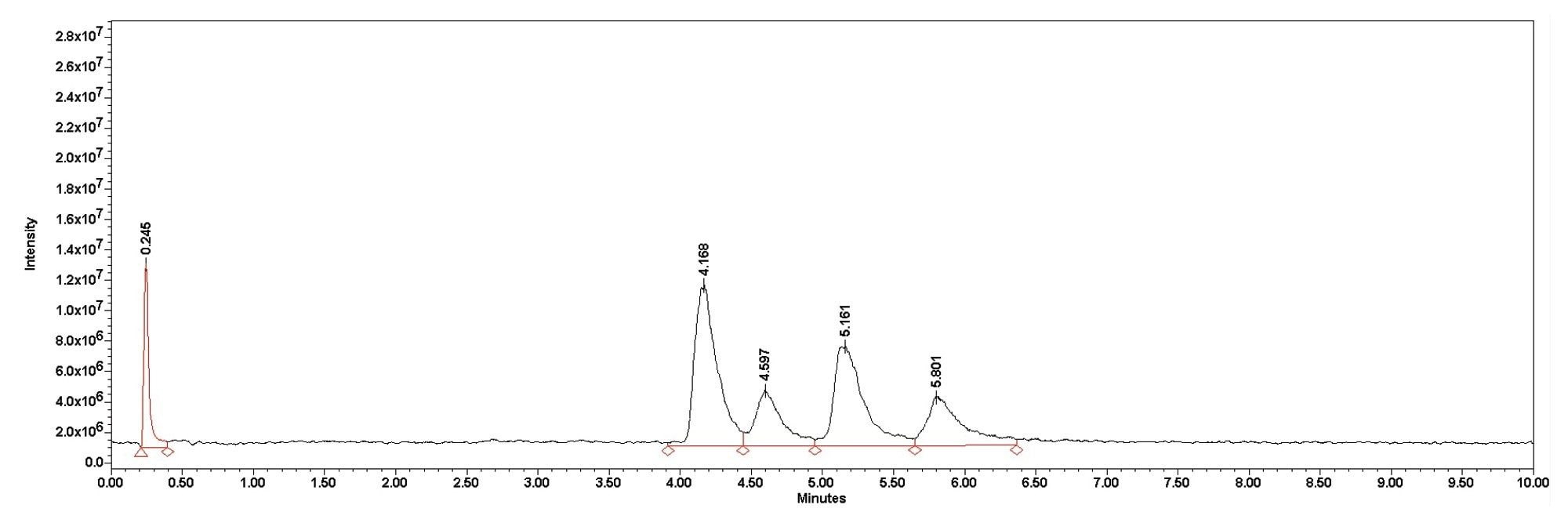
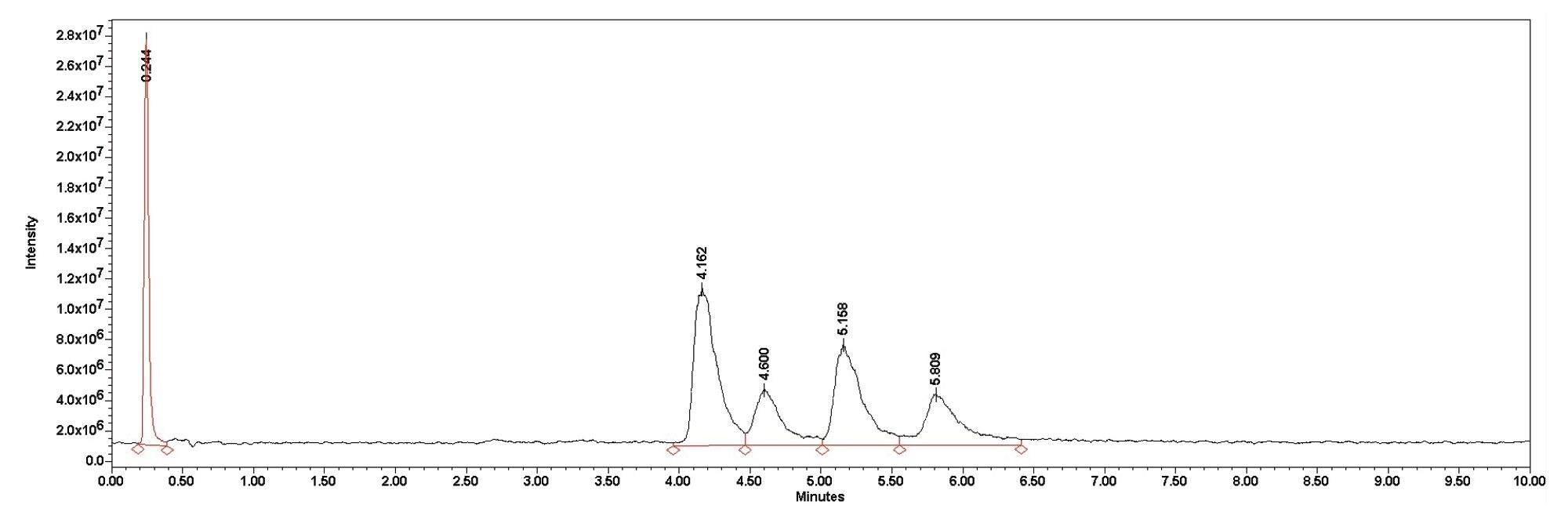
Conclusion
A fast isocratic method using new Atlantis Premier BEH Z-HILIC Column in combination with Waters ACQUITY UPLC H-Class PLUS System with ACQUITY QDa Detector for separation of gentamicin has been created that produces consistent, and reproducible results across multiple batches of packing material. This simple method can quickly separate the four main components of gentamicin (C1, C1a, C2, C2a) all while avoiding the shortcomings of other gentamicin separations like fluorinated pairing agents, complicated pre-analysis derivatization, or long run times.
References
- Masamichi Kobayashi, Michihiko Sone, Masayuki Umemura, Toshitaka Nabeshima, Tsutomu Nakashima & Sten Hellström (2008). Comparisons of Cochleotoxicity Among Three Gentamicin Compounds Following Intratympanic Application, Acta Oto-Laryngologica, 128:3, 245–249, DOI: 10.1080/00016480701558948.
- Wohlfart J, Holzgrabe U. Analysis of Histamine and Sisomicin in Gentamicin: Search for the Causative Agents of Adverse Effects. Arch Pharm (Weinheim). 2021 Dec;354(12):e2100260. doi: 10.1002/ardp.202100260. Epub 2021 Aug 24. PMID: 34427364.
- Kumar P, Rubies A, Companyó R, Centrich F. Hydrophilic Interaction Chromatography for the Analysis of Aminoglycosides. J Sep Sci. 2012 Feb;35(4):498–504. doi: 10.1002/jssc.201100860. PMID: 22282410.
- Stypulkowska, K., Blazewicz, A., Fijalek, Z. et al. Determination of Gentamicin Sulphate Composition and Related Substances in Pharmaceutical Preparations by LC With Charged Aerosol Detection. Chroma 72, 1225–1229 (2010). https://doi.org/10.1365/s10337-010-1763-y.
- White LO, Lovering A, Reeves DS. Variations in Gentamicin c1, c1a, c2, and c2a Content of Some Preparations of Gentamicin Sulphate Used Clinically as Determined by High-Performance Liquid Chromatography. Ther Drug Monit. 1983;5(1):123–6. doi: 10.1097/00007691-198303000-00014. PMID: 6845395.
- Wei J, Shen A, Wan H, Yan J, Yang B, Guo Z, Zhang F, Liang X. Highly Selective Separation of Aminoglycoside Antibiotics on a Zwitterionic Click Te-Cys Column. J Sep Sci. 2014 Jul;37(14):1781–7. doi: 10.1002/jssc.201400080. Epub 2014 Jun 5. PMID: 24798626.
- Fadi L. Alkhateeb, Adam Bengtson, Paul D. Rainville, Simultaneous Separation and Quantification of Aminoglycosides Using Liquid Chromatography and Mass Spectrometry, 2022. Waters Application Note, 720007558.
- McMaster, John Wiley, M. C. Appendix B Solvents and Volatile Buffers for LC/MS. InLC/MS: A practical user's guide (pp. 139–140). essay, 2005.
720007609, April 2022