
This application note summarizes the results of a simple migration of a USP-based HPLC assay to a UPLC-based method.
The migration and consolidation of legacy methods to the Waters ACQUITY UPLC System is a major focus for companies adopting UltraPerformance LC (UPLC) technology. Those companies that invest in the process are rewarded with significant savings in analysis times and operational costs while conserving or improving overall chromatographic performance.
Many laboratories are choosing to redevelop legacy methods during this migration in order to take full advantage of the strengths of the ACQUITY UPLC System, creating even more robust methods in the process. However, some legacy methods may not require full redevelopment; users can still realize significant method improvement by simply transferring their method from HPLC to UPLC.
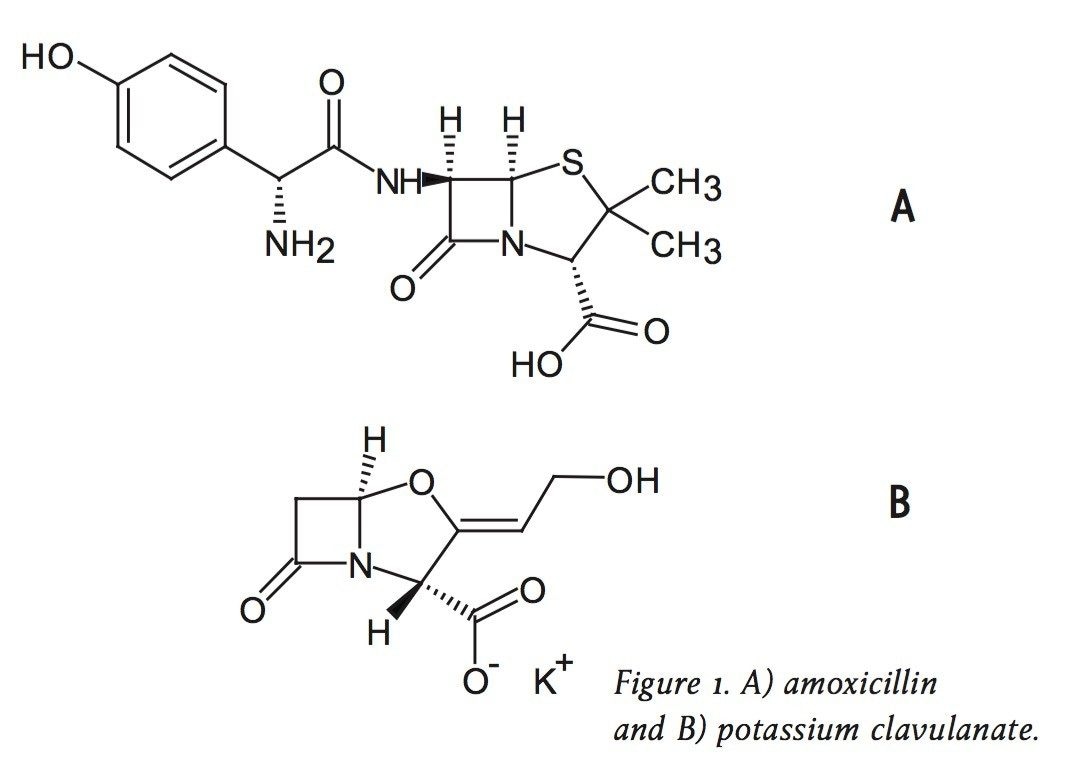
For example, the assay of amoxicillin and potassium clavulanate from an oral suspension1 is an established HPLC method that can be simply migrated to the ACQUITY UPLC with minimum time and effort. Amoxicillin (Figure 1A) is a ß-lactam antibiotic with primary activity against gram-positive bacteria. To broaden the effect of this drug on select gram-negative bacteria and to combat resistant strains, potassium clavulanate (Figure 1B) is added to formulations of amoxicillin to inhibit amoxicillin degradation by the enzyme ß-lactamase.
This application note summarizes the results of a simple migration of a USP-based HPLC assay to a UPLC-based method.
Amoxicillin and potassium clavulanate standards, as well as sodium phosphate, were purchased from Sigma-Aldrich Co. (St. Louis, MO). Methanol was purchased from Fisher (Fair Lawn, NJ). Water used in the study was purified with a Milli-Q Gradient A10 System (Millipore, Billerica, MA). The standard and assay preparations were made according to the USP method.1
The USP HPLC verification was performed on an Alliance 2695 Separations Module equipped with a 2996 Photodiode Array (PDA) Detector. A Waters XBridge C18 5 μm 4.6 x 250 mm Column was selected to satisfy the USP L1 phase requirement.
The UPLC development was performed on an ACQUITY UPLC System consisting of a Binary Solvent Manager (BSM), Sample Manager (SM) and Tunable UV Detector (TUV). A Waters ACQUITY UPLC BEH C18 1.7 μm 2.1 x 100 mm Column was selected for the separation. All instruments were controlled and data collected and analyzed using Waters Empower 2 Software.
The isocratic HPLC method, from the USP, was verified using a sodium phosphate buffer, pH 4.4 in the proportion 95:5 with HPLC grade methanol. A 20 μL injection volume was analyzed on the Alliance HPLC System and detected at 220 nm. No sample or column temperatures were stated in the USP method, so a 15 °C sample temperature and 30 °C column temperature were selected.
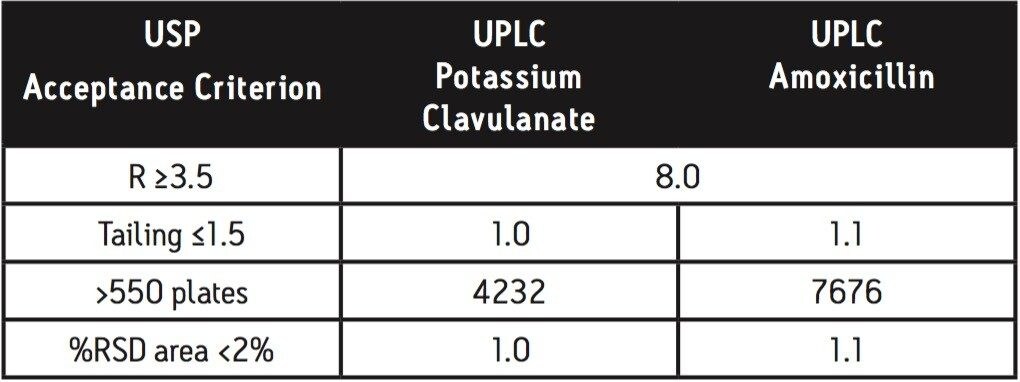
The run time was about 7 minutes, and the flow rate was 1.5 mL/minute. The USP acceptance criteria for this assay were: a resolution of 3.5 or greater for the two components, a USP tailing value of not more than 1.5, a USP plate count of 550 or more and a percent RSD for area of 2% or less for replicate injections. The HPLC assay met the acceptance criteria and was then transferred to the ACQUITY UPLC System.
The verified HPLC method was transferred to UPLC using the Waters ACQUITY UPLC Calculator. As suggested by the calculator, a 1.7 μm, 2.1 x 100 mm ACQUITY UPLC BEH C18 Column was selected for the assay (in order to conserve the L/dp ratio of the HPLC column used in the HPLC assay) with a 1.7 μL injection at a flow rate of 0.40 mL/minute. Detection wavelength (220 nm), mobile phase composition, standard and assay preparations remained unchanged. The sample compartment and column temperatures were maintained at 15 °C and 30 °C, as in the verification. The run time under these new conditions was 3.5 minutes with UPLC compared to 7 minutes for the HPLC method. Figure 2 shows the result of the ACQUITY UPLC assay.
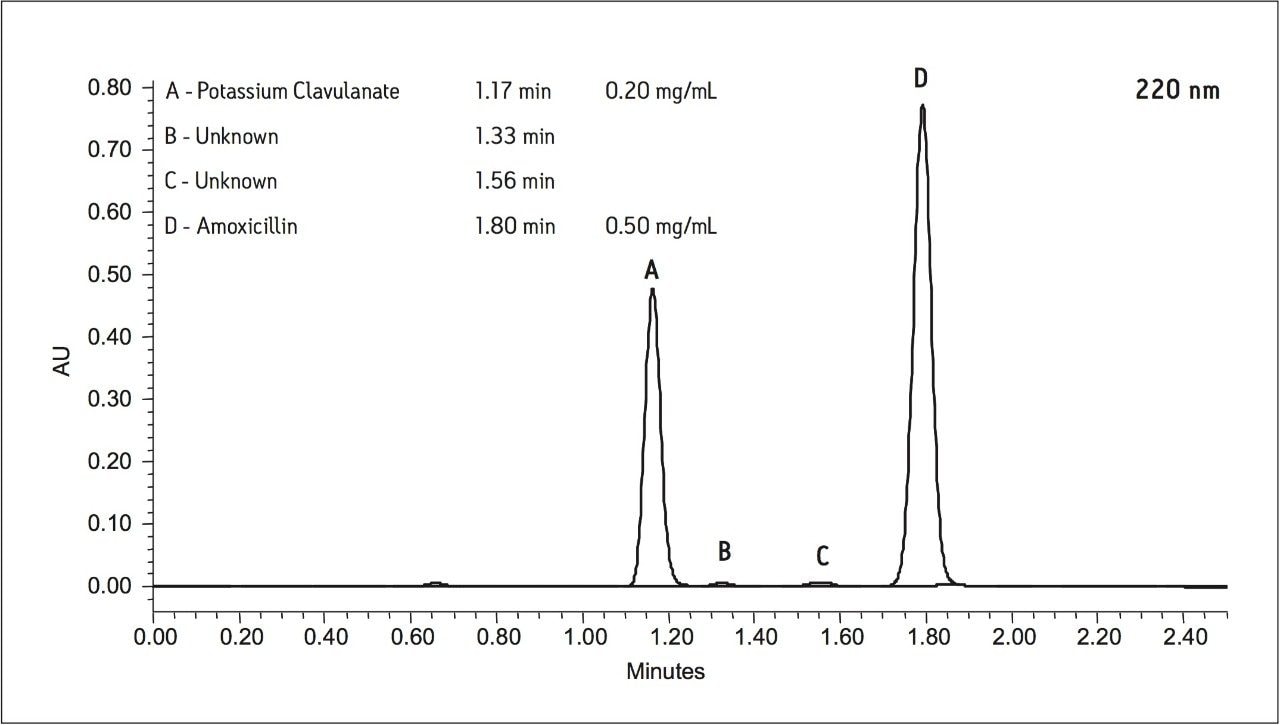
Table 1 compares the assay results to the USP acceptance criteria. This calculator-transferred method easily exceeded the assay acceptance criteria as stated in the USP. The unknown peaks, B and C, were observed in both the original HPLC and transferred UPLC assays.
This transfer was an example of how smoothly an established legacy method can be migrated to the ACQUITY UPLC System. In comparison to the original HPLC assay, the run time was shortened by more than 50 percent (HPLC at 7 minutes; UPLC at 3.5 minutes) and the flow rate reduced by 3.75 times, delivering a savings in time (resulting in an increased sample throughput) as well as overall cost of analysis by reducing solvent usage and waste disposal costs.
Streamlining the migration of this legacy method by using the ACQUITY UPLC Calculator made the transfer quick and simple. In a laboratory already performing the assay, verification would be unnecessary and the transfer process would call for only a single step.
The subsequent validation experiments required following any method transfer could be further facilitated by the application of the Empower 2 Method Validation Manager.
720002043, February 2007