
This application note describes a fast chromatographic method fit for the screening of vanilla extract authenticity. The method has a run time of 5 minutes and demonstrates excellent precision (injection-to-injection, intra-day, and inter-day), linearity, and carryover. A total of 19 commercial extracts were screened including pure, blended, and artificial extracts originating from seven different countries. To test for authenticity the secondary component 4-hydroxybenzaldehyde was quantified and compared to the amount of vanillin. The extracts were also screened for the presence of coumarin. The method was developed on the Waters Breeze 2 HPLC System. This modular system is perfect for routine HPLC analysis particularly for laboratories that require a simple to use chromatographic system.
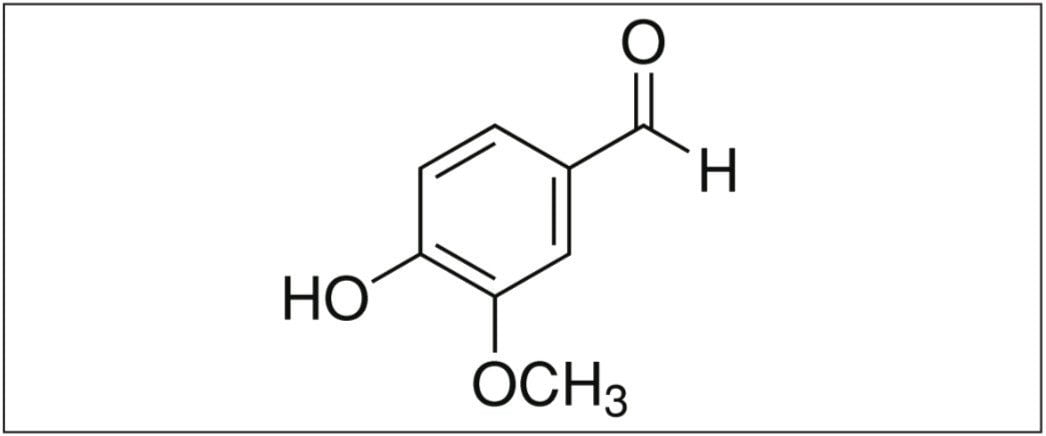
Vanilla is one of the most widely used flavorings in foods and beverages worldwide. It is prepared through the extraction of cured vanilla beans. There are more than 250 organic components in pure vanilla that contribute to its overall flavor and aroma. The main flavor comes from vanillin (4-hydroxy-3-methoxy benzaldehyde, Figure 1). Vanilla originated in Mexico, but varieties are grown in other tropical areas around the world, including Madagascar, Tahiti, and Indonesia. In 2006, the world produced approximately 10,000 tons of vanilla beans, which accounts for less than 1% of the world supply of vanillin.1,2 Most vanillin is synthesized through chemical processes. Two of the most common sources of synthetic or artificial vanilla are lignin vanillin (a by product of the pulp and paper industry) and ethyl vanillin (a coal-tar derivative, which typically has a much stronger flavor than either lignin vanillin or natural vanillin). Pure vanilla is much more expensive than artificial vanilla due to limited quantities of the pure extract (upwards of 100x more expensive for the bulk product). Because of this price differential, counterfeiting can be very profitable. Pure vanilla extract has secondary components, which are not found in synthetic vanilla. These components can be measured at predictable levels and compared to the content of vanillin. Therefore, authenticity can be determined through quantitative separation techniques, such as high performance liquid chromatography (HPLC).3 In addition to counterfeiting, some manufacturers have been known to adulterate the extract with that of the tonka bean, which has a similar essence to the vanilla bean. The tonka bean contains a known toxic compound, coumarin, which has been shown to cause liver damage and is a potential carcinogen.4 Because of this, many countries have banned the use of the tonka bean in vanilla extracts.
This application note describes a fast chromatographic method for the screening of vanilla extract authenticity. The method has a runtime of 5 minutes and demonstrates excellent precision (injection-to-injection, intra-day, and inter-day), linearity, and carryover. A total of 19 commercial extracts were screened, including pure, blended, and artificial extracts originating from seven different countries. To test for authenticity, the secondary component 4-hydroxybenzaldehyde was quantified and compared to the amount of vanillin. The extracts were also screened for the presence of coumarin. The method was developed on the Waters Breeze 2 HPLC System (Figure 2). This modular system is perfect for routine HPLC analysis, particularly for laboratories that require a simple-to-use chromatographic system.

A series of standards (six levels) were diluted from a stock solution (1 mg/mL) of vanillin (Aldrich), ethyl vanillin (SAFC), coumarin (Sigma), and 4-hydroxybenzaldehyde (Aldrich) dissolved in 50/50 water (MilliQ System)/acetonitrile (Fisher Optima Grade). Samples were prepared by diluting 1 mL of commercial vanilla extract in 100 mL volumetric flasks with 50/50 water/acetonitrile. The chromatographic method was as follows:
|
LC system: |
Breeze 2 HPLC 1525 Binary HPLC Pump with Column Heating 2707 Autosampler 2489 UV/Vis Detector |
|
Data: |
Breeze 2 Software |
|
Column: |
Atlantis T3 C18 4.6 mm x 100 mm 3 μm |
|
Injection volume: |
10 μL |
|
Temperature: |
40 °C |
|
Flow rate: |
1.5 mL/min |
|
Dial-a-mix: |
70/30 water/acetonitrile (isocratic) |
|
Needlewash solvent: |
70/15/15 acetonitrile/isopropanol/water |
|
Detection: |
Detection wavelength: 275 nm |
|
Data rate: |
5 Hz |
|
Time constant: |
0.4 s (normal) |
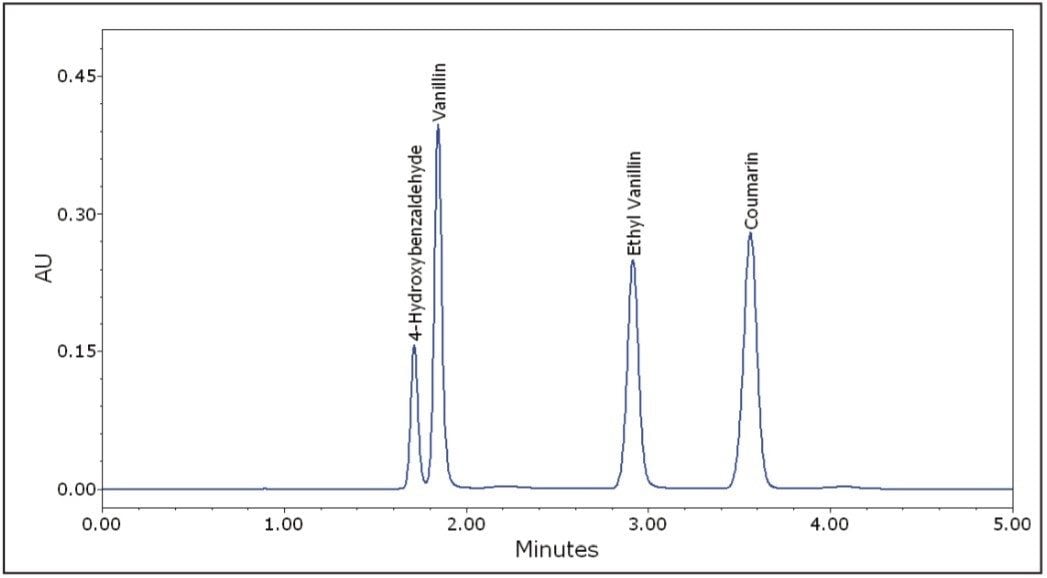
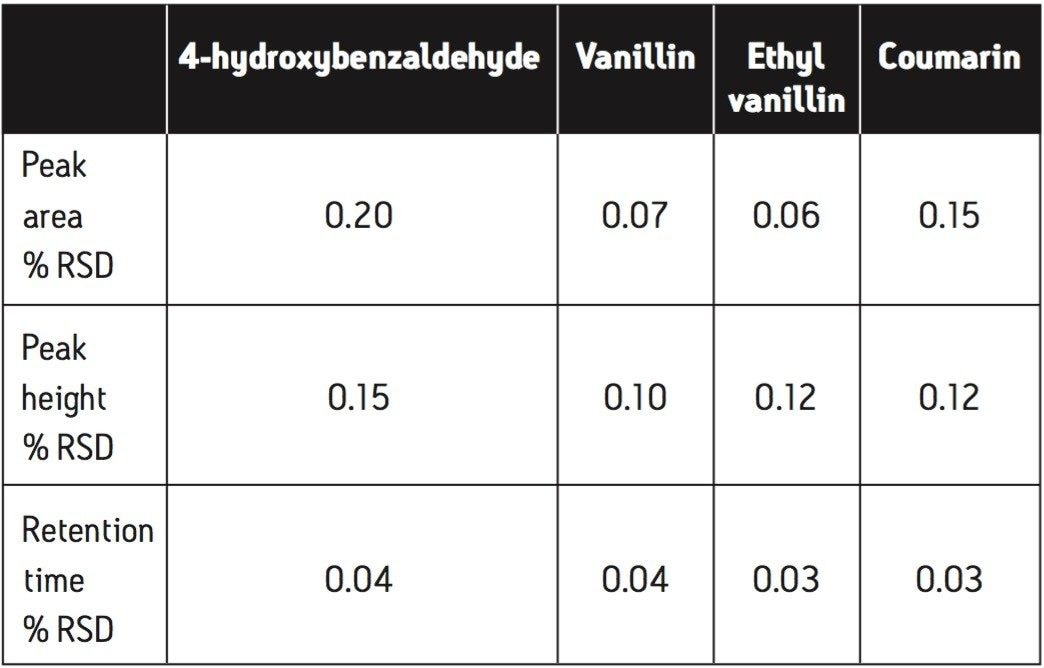
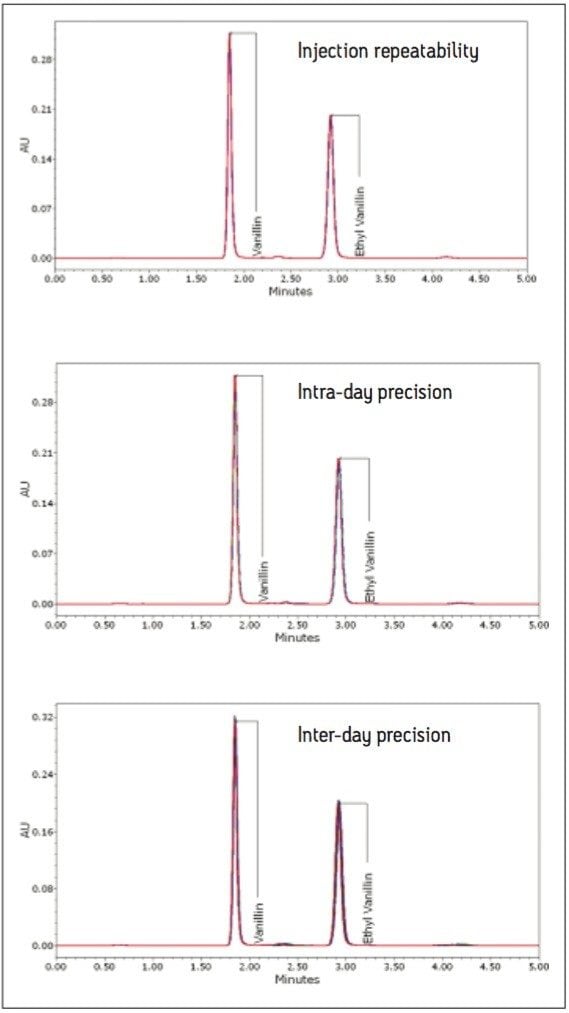
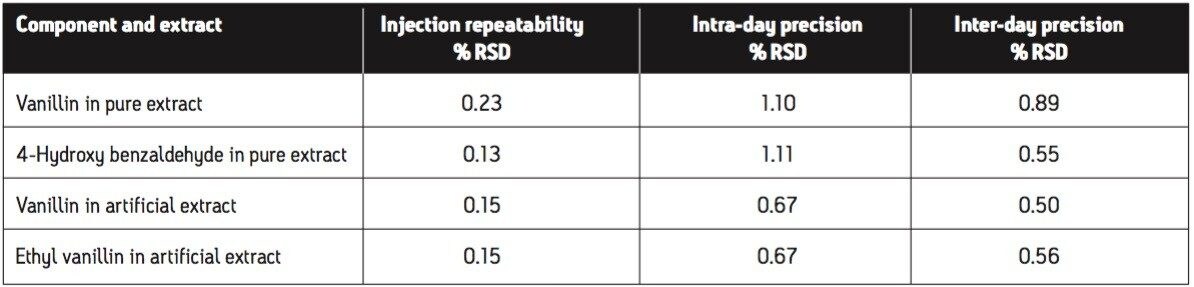
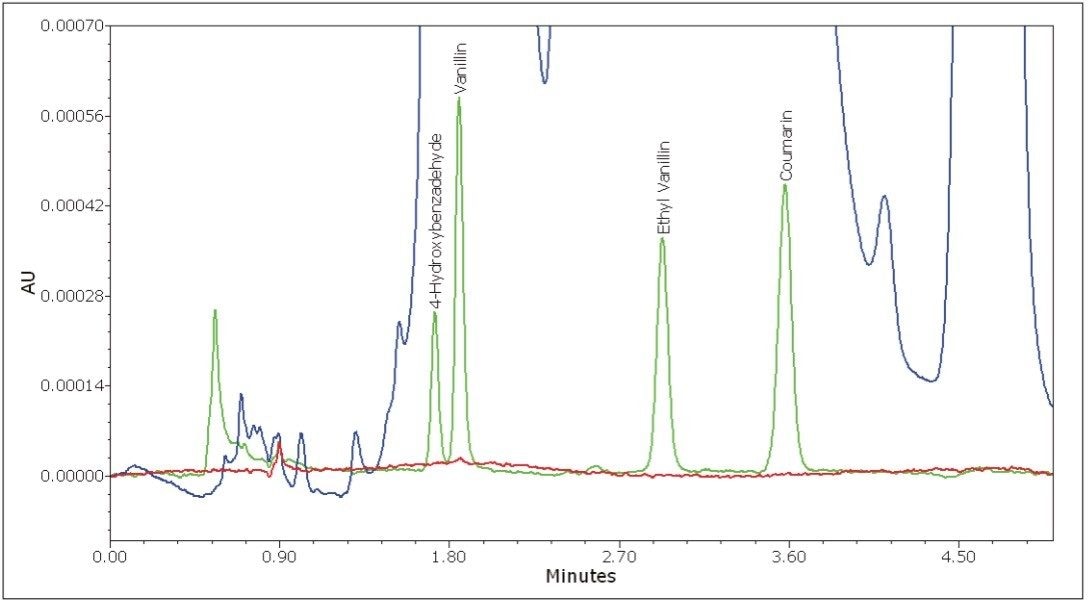
The isocratic separation of 4-hydroxybenzaldehyde, vanillin, ethyl vanillin, and coumarin was achieved in 5 minutes (Figure 3). To assess repeatability for each of the four components, six replicate injections of an intermediate level standard were performed. Excellent repeatability for retention time, peak area, and peak height was observed for all four components (Table 1).
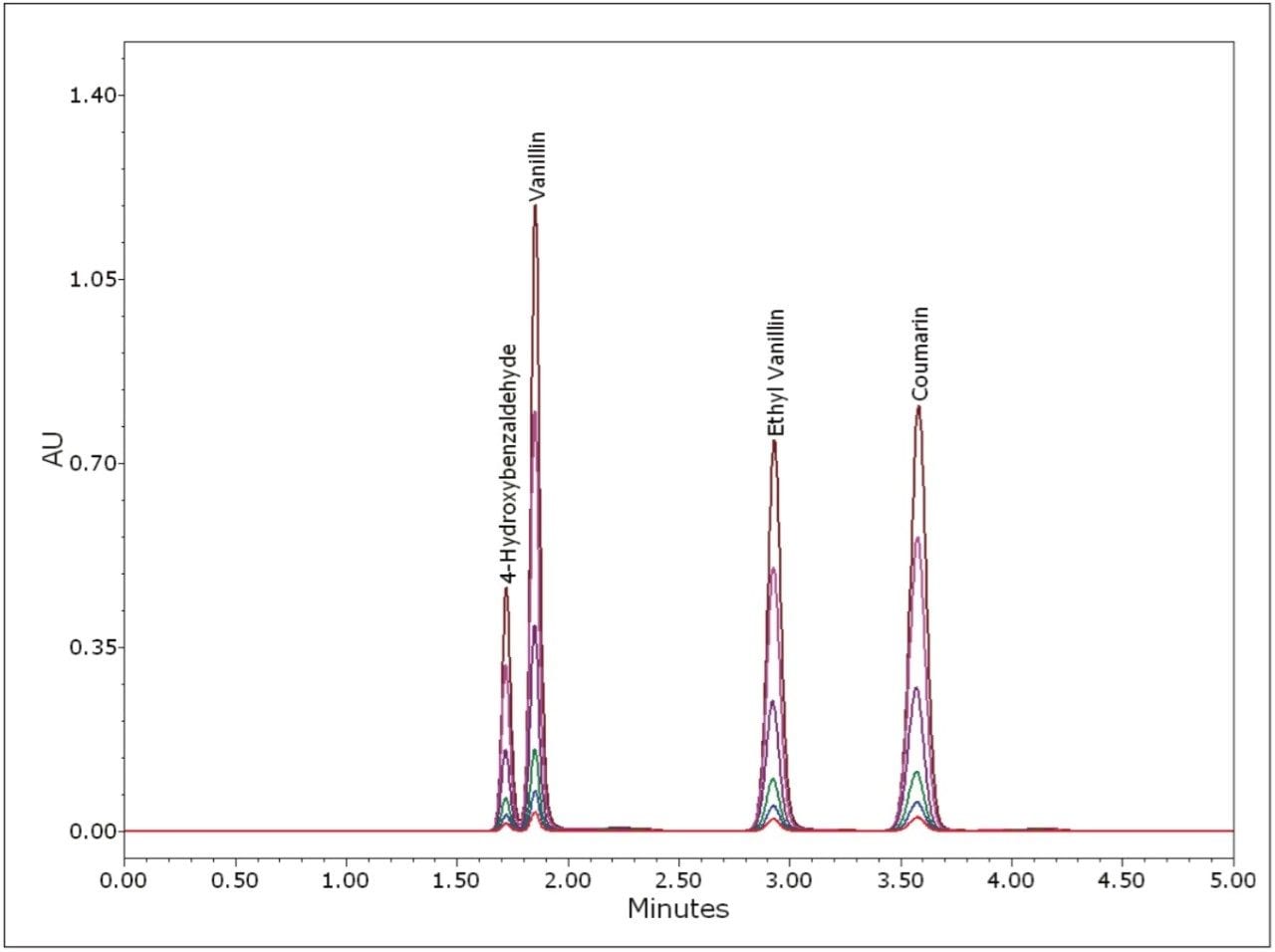
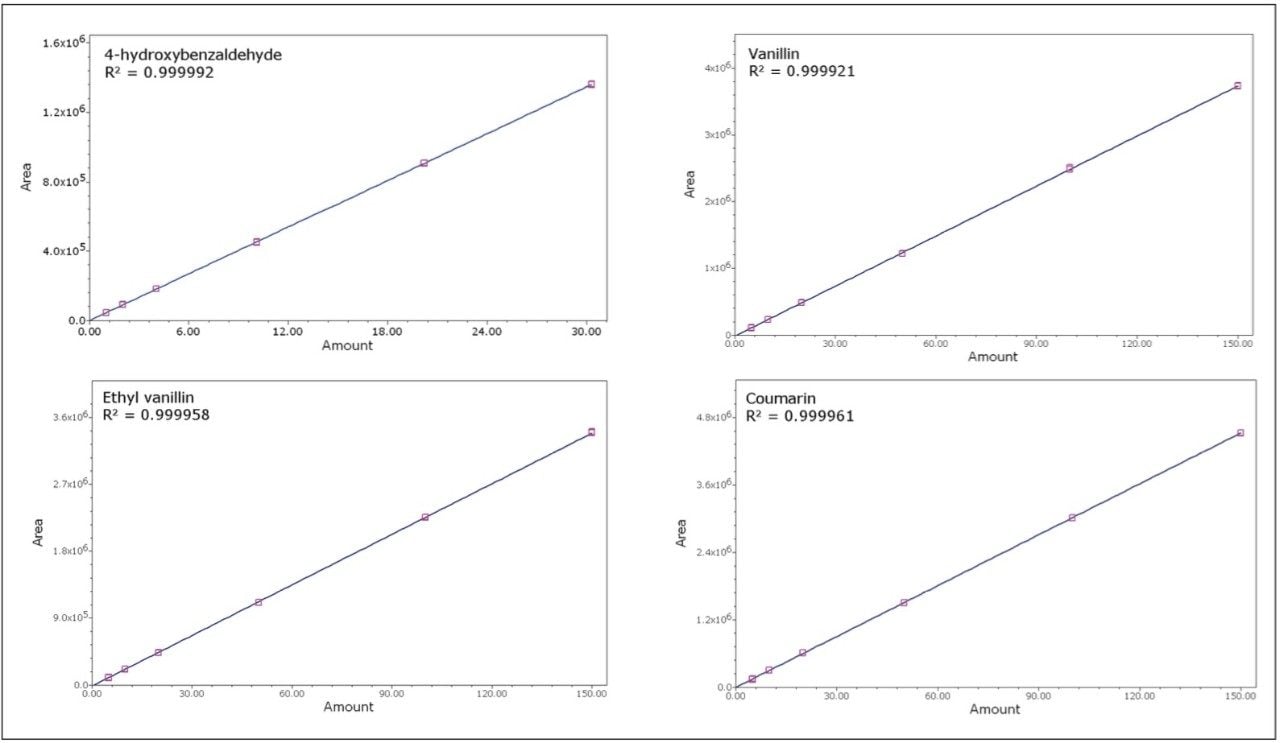
To assess the method linearity, standards were prepared at six levels. An overlay of injections at each level is displayed in Figure 4. Note that there is no peak distortion at the higher levels, which indicates that the injection load is within the column loading capacity. A range covering more than one order of magnitude was necessary to span the concentration range used in all of the pure, artificial, and blended extracts. Because of this, a 1/x weighting was used for the calibration curves to ensure good quantitative accuracy for the lower level components. For each curve, standards were injected in duplicate. Excellent linearity was observed with correlation coefficients in excess of 0.9999 for all four components. The resulting calibration curves are displayed in Figure 5. The excellent precision of the 2707 Autosampler and the large linear dynamic range of the 2489 UV/Vis Detector provide for methods with excellent chromatographic linearity.
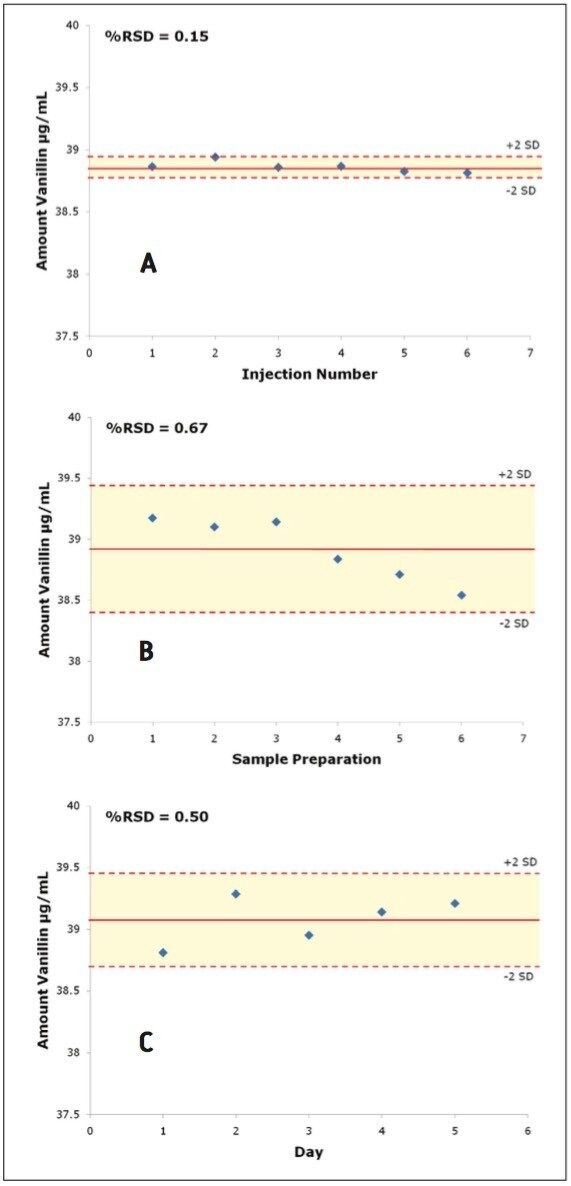
Precision of the method was assessed on two samples: a pure vanilla extract (Extract C) and an artificial vanilla extract (Extract O). A calibration curve was prepared by injecting six standard levels in duplicate and then the amount for each prepared sample was calculated. Three levels of precision were assessed: 1) injection repeatability (one sample preparation injected six times); 2) intra-day precision (six sample preparations injected in triplicate); and 3) inter-day precision (each day for 5 days, two new sample preparations were injected in triplicate). For the inter-day precision test, standards were prepared fresh each day, whereas for the intra-day precision test, the same calibration curve was used for all six preparations. Figure 6 displays overlays from the three precision experiments. Theoverlays are virtually identical demonstrating the excellent overall precision of this method on the Breeze 2 HPLC System. The calculated amounts for each of the three precision experiments with the standard deviation bands at ±2 sigma are shown in Figure 7. The calculated values for all of the extracts can be found in Table 2. Excellent precision for the quantification of vanillin was observed for all of the experiments, well below typical acceptance criteria 2.00% RSD. The injection to-injection repeatability was only 0.23% and 0.15% RSD for vanillin in the pure and artificial extracts respectively. This value is well below the inter-day and intra day precision values indicating it was only a small contributor to the overall method precision. The 2707 Autosampler of the Breeze 2 HPLC System is a very precise autosampler and is well-suited for this type of day-to-day screening or quality control applications.
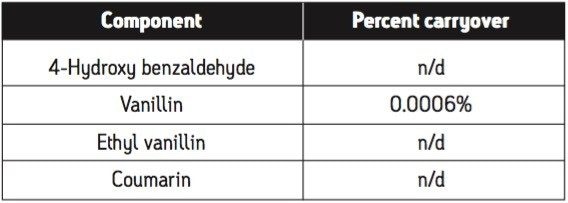
Carryover was assessed using the highest concentration standard (150 μg/mL). To measure carryover, the following injection sequence was used: two pre-injection blanks (to ensure no signal was observed at the retention time of the compounds of interest); six injections of the carryover standard (prepared at 0.05% of the high concentration standard); six injections of the high concentration standard (150 μg/mL); three blank injections from three separate vials. The percent carryover is assessed by comparing the signals in the blank injections to those in the carryover standard. The data is displayed Figure 8. Using the default wash settings of the 2707 Autosampler, the carryover measured for the vanilla screening standard was well below instrument specification of 0.05%. Table 3 displays the calculated carryover amounts for each of the blank injections.
A total of 19 vanilla extracts were screened for authenticity. A variety of extracts were selected, including six pure domestic extracts; five pure imported extracts; six artificial extracts; and two blended extracts. Table 4 displays the screening results for all 19 vanilla extracts.
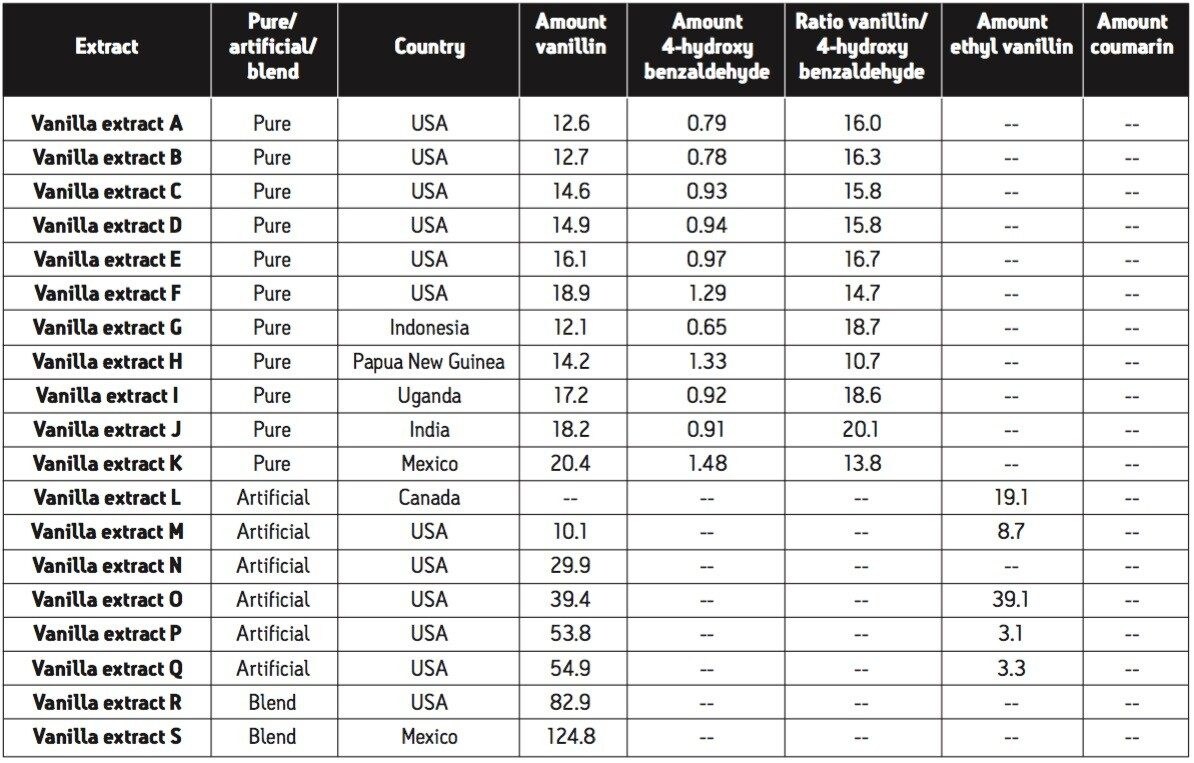
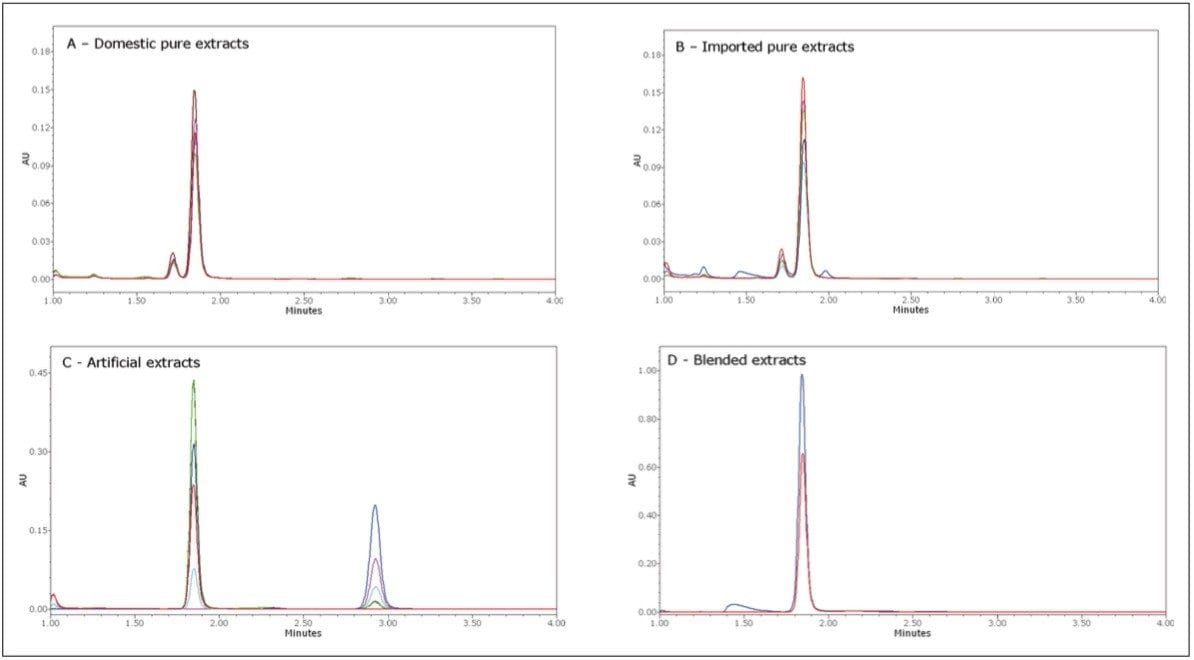
To screen pure extracts for authenticity, methods have been cited in the literature that examine the amount of various secondary components in the extract compared to the amount of vanillin.3 The secondary components that have been used include vanillic acid, 4-hydroxybenzoic acid, and 4-hydroxybenzaldehyde. For this analysis, 4-hydroxybenzaldehyde was chosen as the marker for authenticity due to its relative retention time. The domestic pure vanilla extracts had similar vanillin content ranging from 12.6 to 18.9 μg/mL and had very similar ratios of vanillin/4-hydroxybenzaldehyde ranging from 14.7 to 16.0 μg/μg. This could indicate a very similar extraction process or a common source of cured vanilla beans. An overlay of the resulting chromatograms for these pure extracts is displayed in Figure 9A. The U.S. Food and Drug Administration regulates the process for all extracts. In order to label an extract as pure, the extraction solvent must contain at least 35% alcohol.5 Most of the domestic pure extracts did indicate that 35% alcohol was used for the extraction. The imported vanilla extracts had a similar range for the content of vanillin at 12.1 to 20.4 μg/mL but had a larger range for the ratio of vanillin/4-hydroxybenzaldehyde at 10.7 to 20.1 μg/μg. An overlay of the resulting chromatograms for these imported pure extracts is displayed in Figure 9B. This is not unexpected as the type, growing conditions, curing process, and extraction process will likely vary more significantly for these extracts than for the domestic vanilla extracts. Although the range for the vanillin/4-hydroxybenzaldehyde range was larger for the imported vanilla extracts, it is within the range that has been reported by other authors utilizing HPLC for vanilla authenticity screening.3 Coumarin and ethyl vanillin were not detected in any of the pure extracts. The screening results of the 11 vanilla extracts labeled as pure indicates that these are indeed authentic extracts.
The artificial extracts all varied greatly as to the amount of vanillin or ethyl vanillin and all contained more total flavoring than the pure extracts (ethyl vanillin has a much stronger aroma/flavor than vanillin). None of the artificial extracts contained the banned compound coumarin. An overlay of the resulting chromatograms is displayed in Figure 9C. The absorbance scale is 2.5x higher than that of the pure vanilla extracts.
In the blended extracts that were tested, 4-hydroxybenzaldehyde was not detected indicating that no pure vanilla extract was present in the product. Instead, the vanillin in these extracts appears to be only from an artificial source. The resulting chromatography for the blended extracts is displayed in Figure 9D. The banned substance coumarin was not observed in either of these extracts.
720002877, December 2008