For research use only. Not for use in diagnostic procedures.
This application note describes the optimization of the methodology to improve the speed, cost and useability for routine quantitative analysis of aβ peptides in hCSF.
Herein we demonstrate the suitability of the Xevo TQ-S micro, a cost-effective platform for biomarker quantification, for the accurate quantification of multiple aβ peptides (1–38, 1–40, 1–42) extracted from hCSF at reported concentrations of 0.1–10 ng/mL, while using a decreased sample volume (100 μL) with respect to previous application notes.
Amyloid beta (aβ) peptides, compounds involved in Alzheimer’s disease pathogenesis, have been targeted as Alzheimer’s biomarkers and almost exclusively quantified using immunoassay techniques.1,2,3 These techniques are known to be time consuming, subject to cross-reactivity, and with high batch to batch variation. To overcome these challenges, Waters developed a fast and flexible Solid Phase Extraction-Liquid Chromatography method coupled to tandem Mass Spectrometry (SPE LC-MS/MS) for the quantification of multiple aβ peptides in human CSF (hCSF) to support preclinical research and biomarker discovery.4,5 This application note describes the further optimization of the methodology to improve the speed, cost and useability for routine quantitative analysis of aβ peptides in hCSF.
Herein we demonstrate the suitability of the Xevo TQ-S micro, a cost-effective platform for biomarker quantification, for the accurate quantification of multiple aβ peptides (1–38, 1–40, 1–42) extracted from hCSF at reported concentrations of 0.1–10 ng/mL, while using a decreased sample volume (100 μL) with respect to previous application notes.4,5 The method performance meets the FDA Bioanalytical Method Validation Guidelines for the accurate quantification of endogenous aβ peptides in hCSF.6 Reproducible and accurate quantification of aβ 1–42 Certified Reference Material was achieved with this method.7 The automation compatibility of the sample preparation workflow was also demonstrated using a Tecan automated pipetting platform.
|
Recommended UPLC hardware components: |
ACQUITY UPLC I-Class configured with fixed loop sample manager |
|
Sample loop volume: |
100 μL (p/n: 430004209) |
|
Needle: |
20 μL (p/n: 700005927) |
|
Injection mode: |
Partial loop with needle overfill |
|
Binary solvent mixer: |
100 μL (p/n: 205000854) |
|
Column: |
ACQUITY UPLC Peptide BEH C18, 300Å, 1.7 μm, 2.1 × 150 mm (p/n 186003687) |
|
Column temp.: |
55 °C |
|
Sample temp.: |
15 °C |
|
Injection volume: |
40 μL |
|
Flow rate: |
0.2 mL/min |
|
Mobile phase A: |
0.3% NH4OH in H2O |
|
Mobile phase B: |
90:10 (v/v) ACN:Mobile phase A |
|
Strong needle wash: |
80:20 (v/v) ACN:IPA +10% NH4OH (600 μL) |
|
Weak needle wash: |
95:5 (v/v) H2O:ACN + 0.3% NH4OH (600 μL) |
|
Time(min) |
Profile |
Curve |
|
|
%A |
%B |
||
|
0.0 |
90 |
10 |
6 |
|
1.0 |
90 |
10 |
6 |
|
6.5 |
55 |
45 |
6 |
|
6.7 |
55 |
45 |
6 |
|
7.0 |
90 |
10 |
6 |
|
9.0 |
90 |
10 |
6 |
|
Capillary voltage: |
2.5 V |
|
Desolvation temp.: |
650 °C |
|
Cone gas flow: |
150 L/hr |
|
Desolvation gas flow: |
1000 L/hr |
|
Collision cell pressure: |
2.6 × 10(-3) mbar |
|
MRM transitions monitored: |
ESI+: See Table 1 |
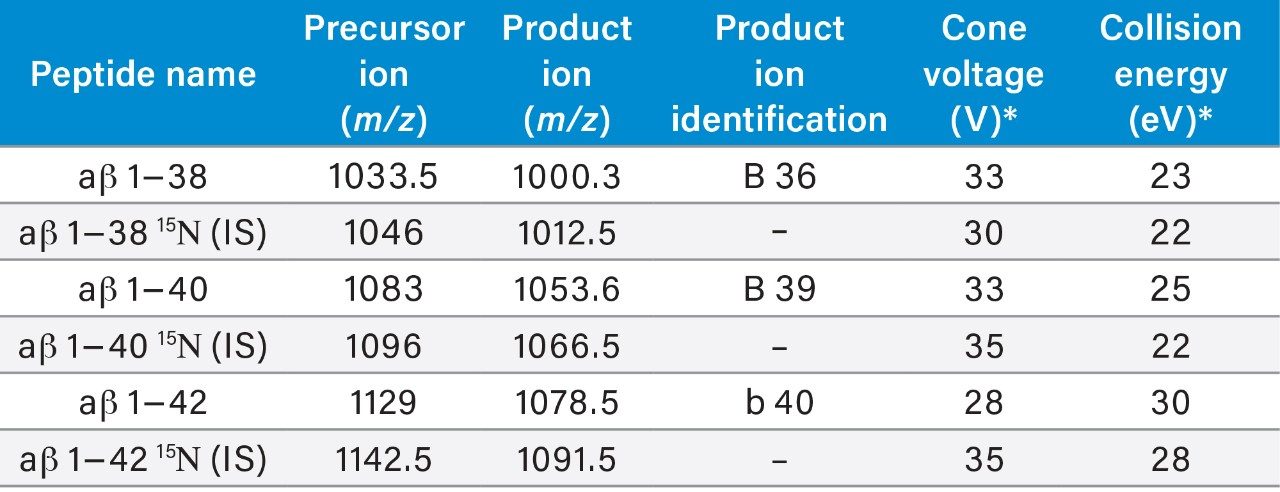
Table 1. Multiple reaction monitoring (MRM) transitions and MS conditions for the aβ peptides and their corresponding 15N labeled internal standards.
*Mass positions, cone voltage, and collision energy optimized during instrument tuning.
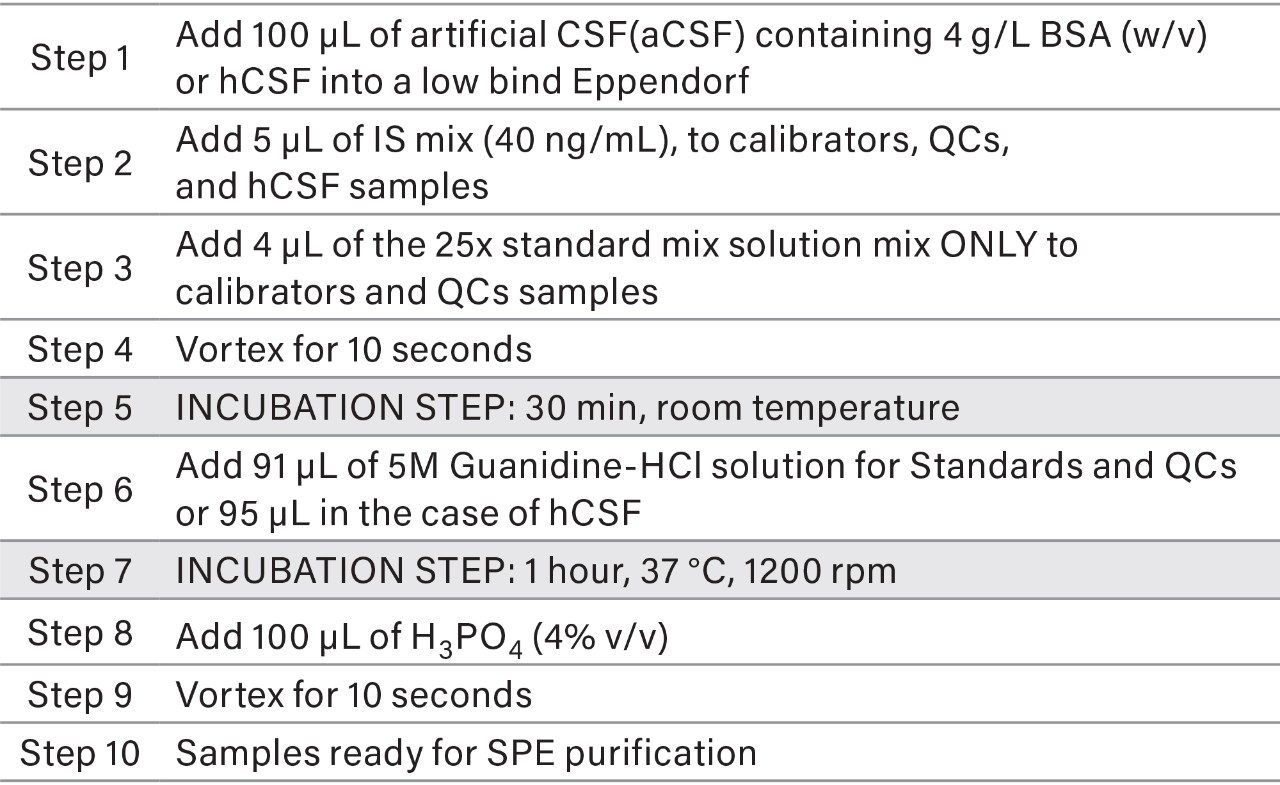
Calibrators, quality controls (QC) and hCSF sample preparation and pretreatment
To prepare calibration standards, QCs and hCSF samples, the procedure described in Table 2 was followed. All standard and internal standard (IS) mixes were prepared in spiking solutions composed of 50:50:1 Acetonitrile:Water:NH4OH containing 0.05% rat plasma. The standard mix solutions contained all 3 isoforms of aβ peptides at 25× the desired calibrator/QC concentration, while the IS mix contained all 3 isoforms of 15Ν aβ peptides. Final concentrations of prepared standards ranged from 0.1 to 10 ng/ml in the case of standards and QCs for native aβ peptides, while IS peptide mix was prepared at 40 ng/mL. Samples were prepared and injected in triplicate, while standards and QCs were prepared and injected in duplicate.
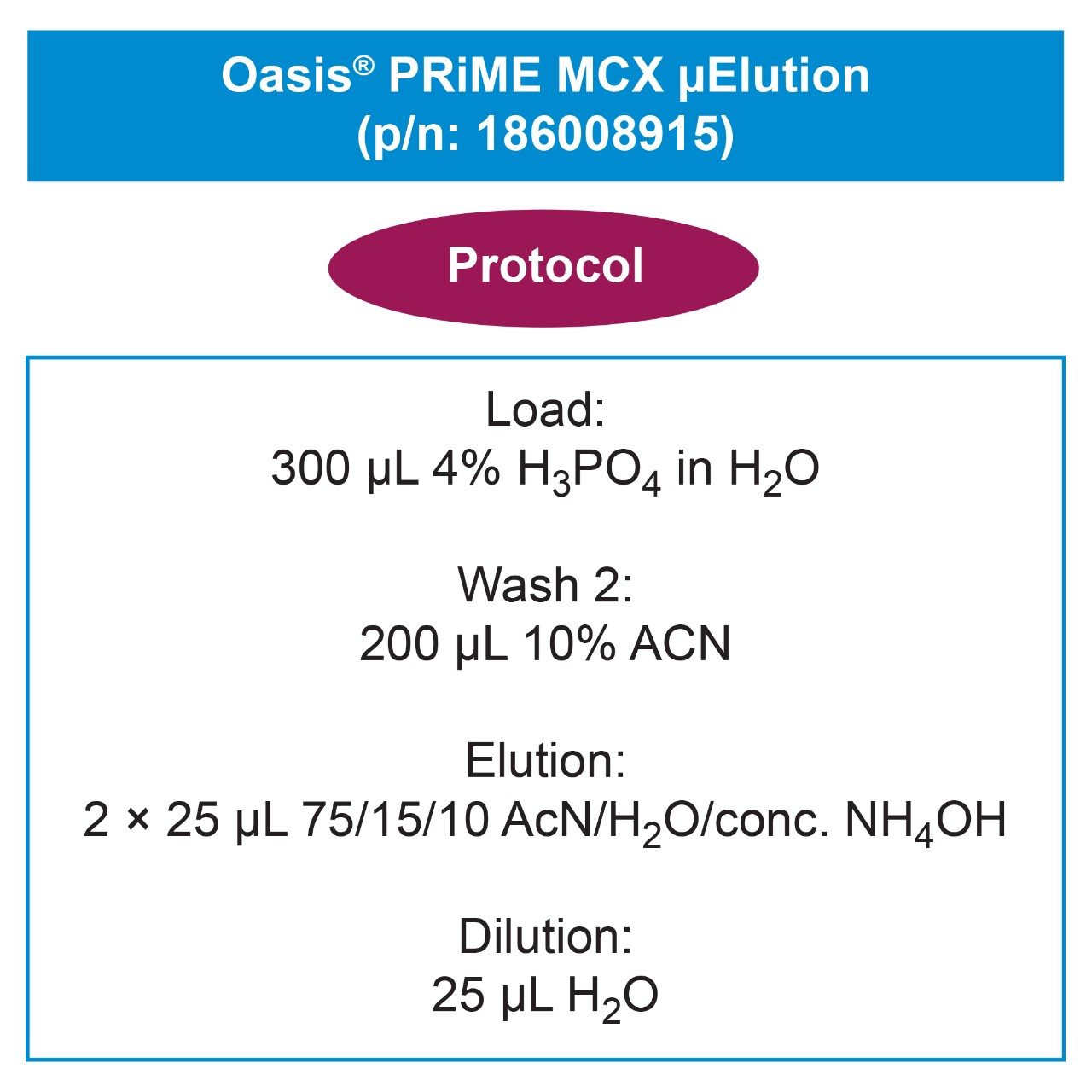
The pretreated samples were extracted according to the protocol described in Figure 1 using Oasis PRiME MCX in the 96-well μElution format and performed on the Tecan Freedom EVO 100/4 automated pipetting platform. All solutions used for extraction were made up by volume.
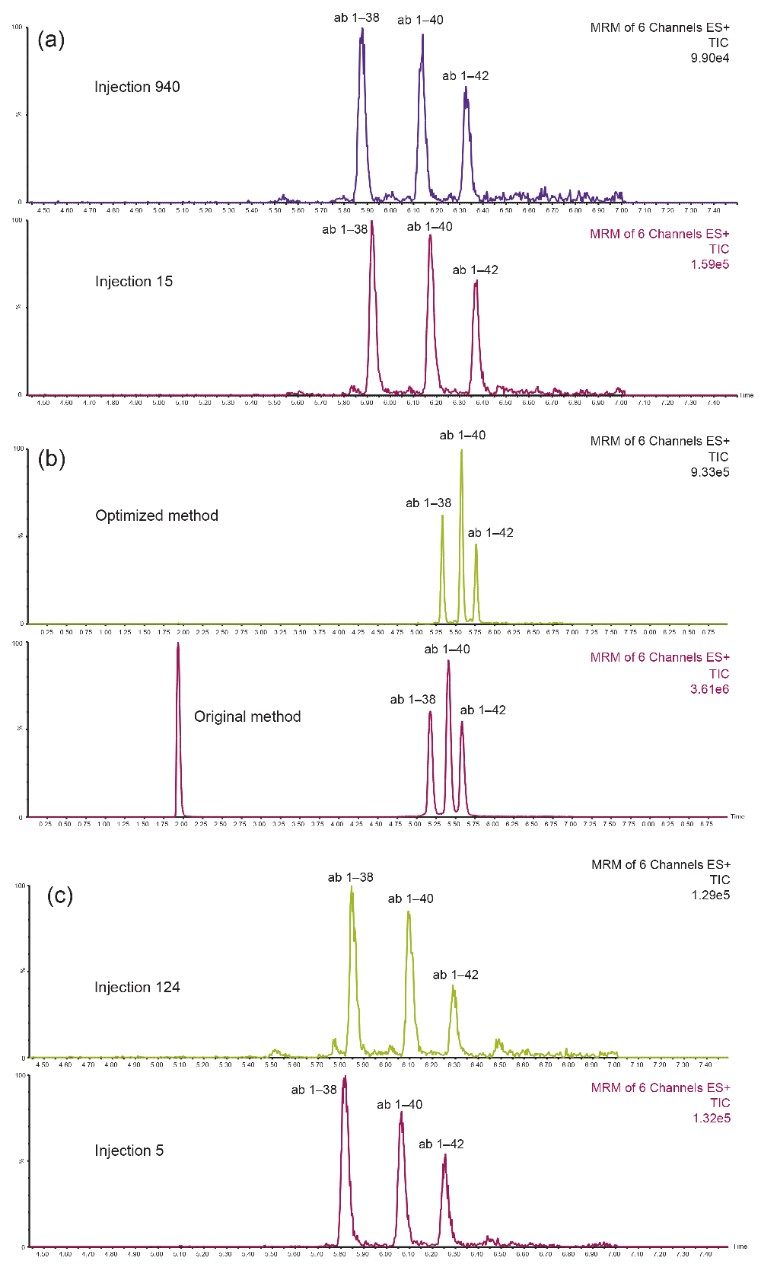
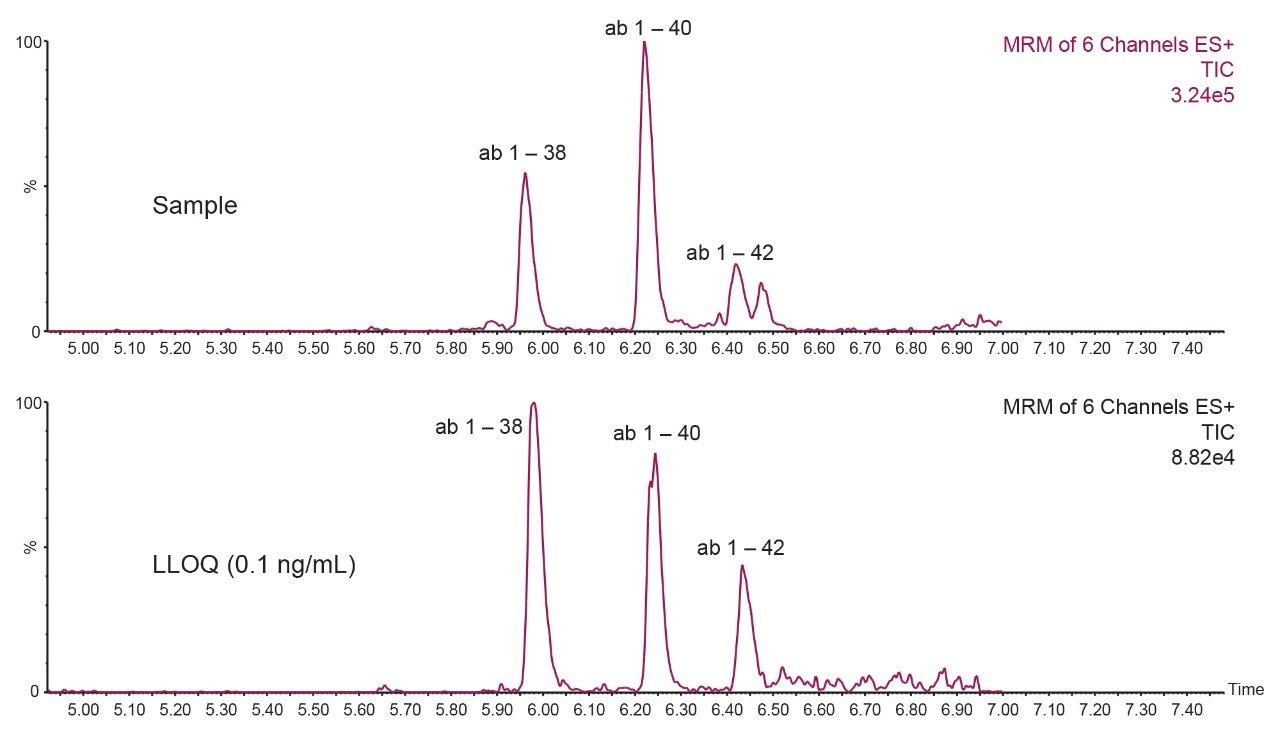
Chromatographic separation of the aβ peptides was achieved using an ACQUITY UPLC Peptide BEH C18 (300Å, 1.7 μm, 2.1 × 150 mm) Column with the ACQUITY UPLC I-Class System in the fixed loop sample configuration. A linear gradient using 10–45% mobile phase B over 5.5 minutes was employed.4,5 A minor modification to the original application with the addition of a 2 min hold step using initial conditions (90% mobile phase A) at the end of the gradient increased the column life time up to 800 injections (Figure 2, Panel a).4 Furthermore, the modification of the weak needle wash solvent (acetonitrile percentage decreased from 10% to 5% while maintaining NH4OH at 0.3%) and strong wash solvent (decreasing IPA percentage from 40% to 20%while maintaining NH4OH constant at 10%), resulted in the elimination of carryover (Figure 2, Panel b). Using this modified method, samples were shown to be stable for 5 days in the auto sampler, while mobile phase stability at room temperature was determined to be 3 days. In addition, when testing 150 injections in one run no appreciable loss of peptide signal was seen, further demonstrating the robustness of this developed method for high throughput analyses (Figure 2, Panel c). The improvements in LC optimization combined with Xevo TQ-S micro MS analysis enabled a lower limit of quantification for this assay of 0.1 ng/mL and the ability to readily detect and quantify endogenous aβ levels from hCSF (Figure 3).
SPE extraction of the aβ peptides was achieved using Oasis PRiME MCX, a mixed-mode sorbent, in the μElution format using the extraction procedure shown in Figure 1. For peptides, SPE sample preparation in the micro elution format is ideal. It provides rapid sample cleanup, high recovery, sample concentration without the need for sample evaporation, and helps ensure peptide solubility throughout the extraction process. Due to the water wettable nature of the Oasis PRiME sorbents, we were able to eliminate the conditioning and equilibration steps, reducing time and number of steps. In addition, Oasis PRiME MCX is designed to yield highly consistent flows across cartridges and plates, making processing time exceptionally reproducible.
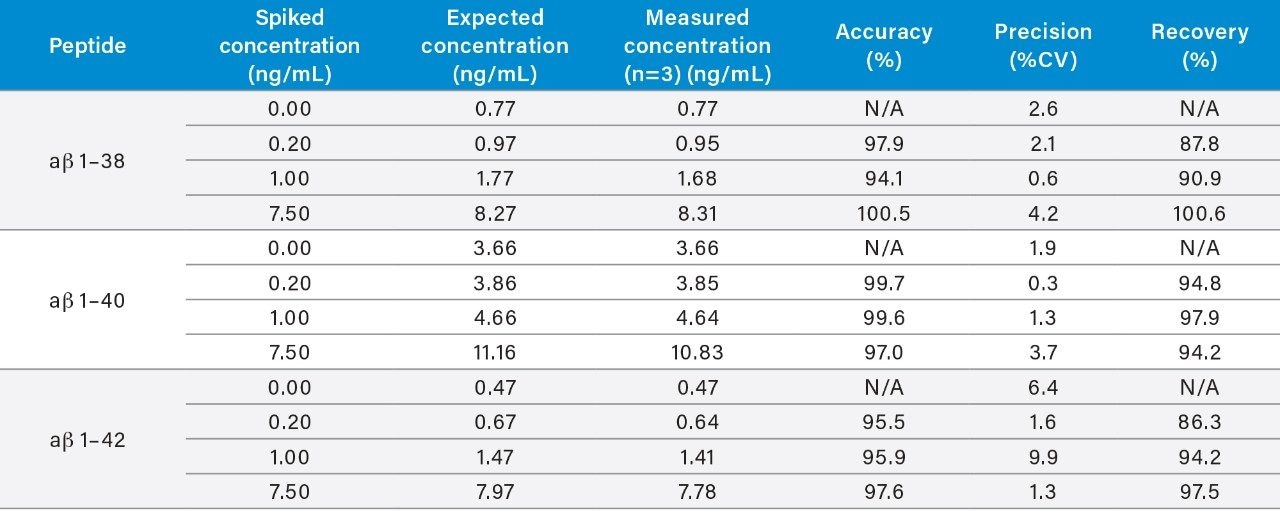
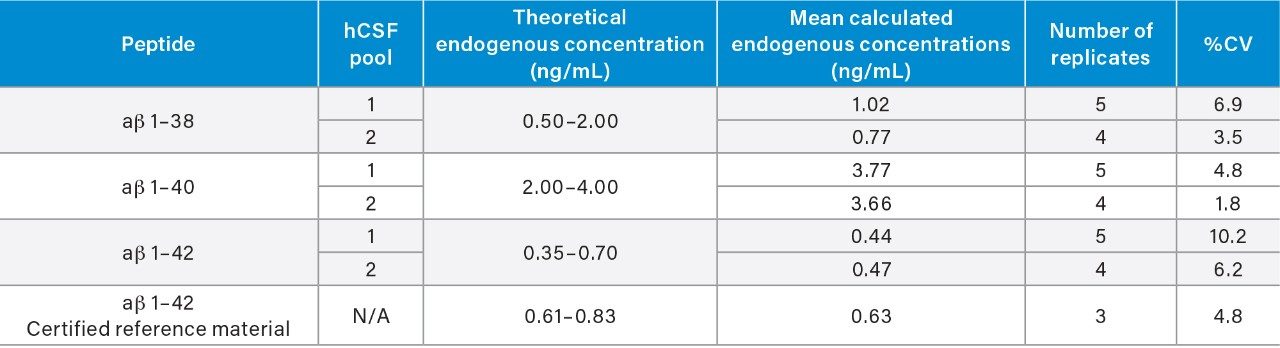
Use of the Oasis PRiME MCX SPE sorbent and the described protocol provided excellent recovery and selectivity for the extraction of the aβ peptides from hCSF, eliminating other high abundance endogenous polypeptides and matrix interferences (Figure 4). A summary of sample recoveries and quantitative performance, for unspiked, low-level QC (LQC), mid-level QC (MQC), and high-level QC (HQC) is highlighted in Table 3. Quantitative performance was excellent with mean (N=3) recovery and accuracies values between 86.3–100.6% and 95.5–100.5%, respectively and precision values (CVs) ≤10%. In addition to QC performance, analysis of aβ concentrations was performed from two sources of pooled hCSF, as well as for the concentration determination for the ERM reference material (CRM) of aβ 1–42.
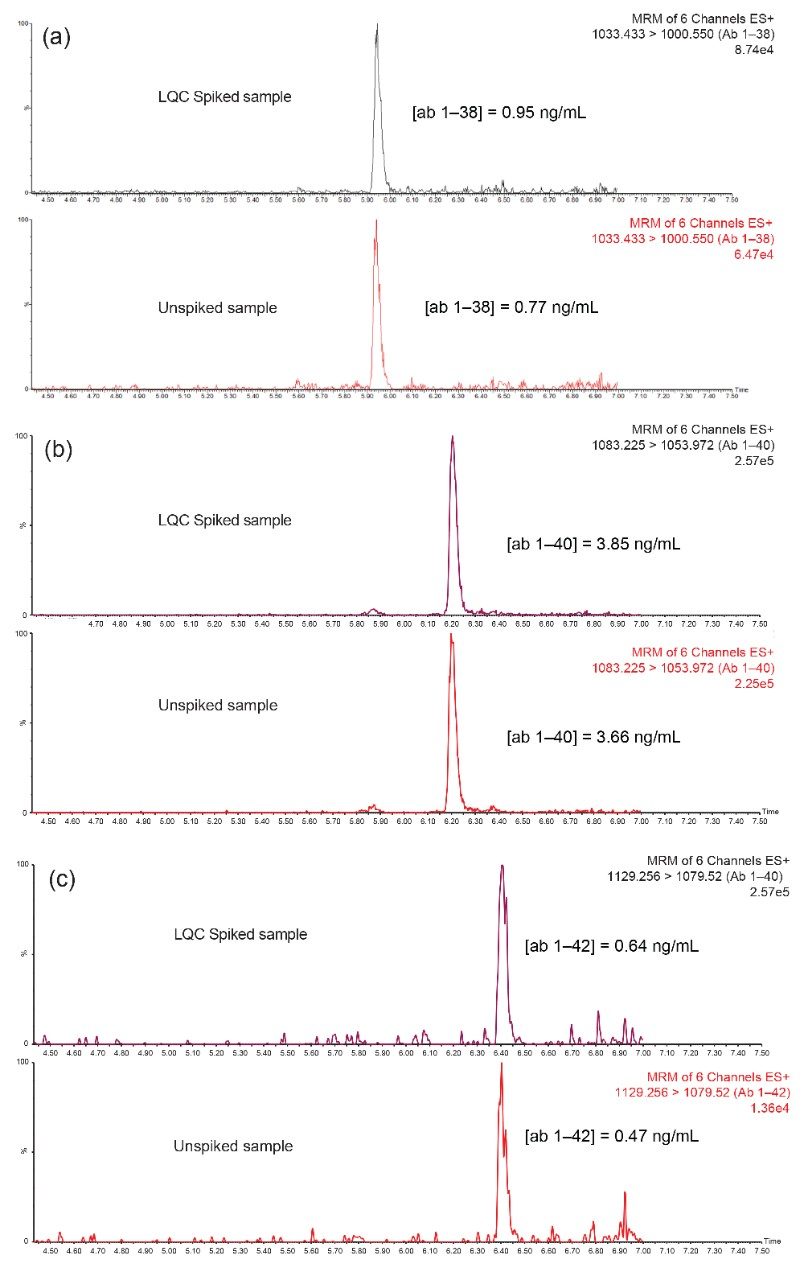
Results from this analysis were in good agreement with reported theoretical values (Table 4). Representative extracted ion chromatograms (EIC) of the 1–38, 1–40, and 1–42 ab isoforms comparing endogenous level (unspiked hCSF) and hCSF spiked with low level QC (0.2 ng/mL) are shown in Figure 4, Panels a–c. Remarkably, decreasing sample size two-fold did not have any effect on the SPE extraction or analytical parameters compared to previous works.4,5
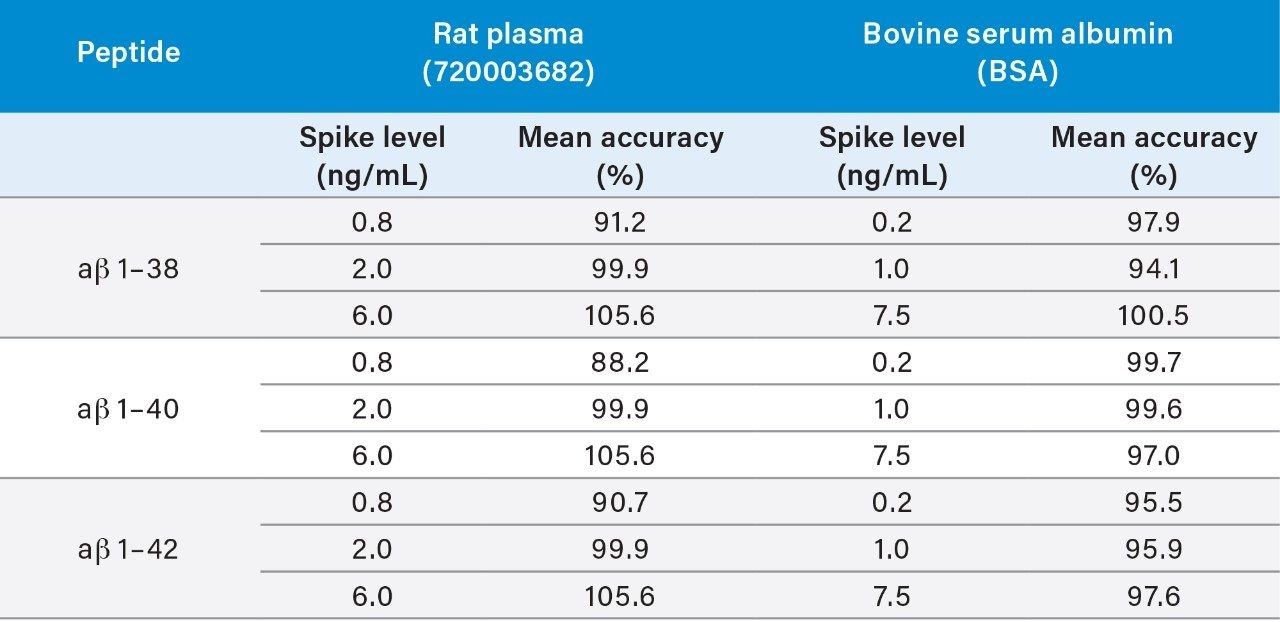
During initial method development, a high degree of non-specific binding (NSB) was observed when aβ peptides were extracted from artificial CSF. Thus, 5% rat plasma was added as a carrier protein to eliminate the NSB.4,5 To simplify the analysis, reduce cost, increase assay robustness and eliminate lot variations in rat plasma, bovine serum albumin (BSA) was assessed as an alternative carrier protein. For this assessment LQC, MQC, and HQC samples were prepared in aCSF containing BSA (4 g/L) and were extracted using the extraction protocol (Figure 1). Mean accuracies of these results were excellent, from 94.1–100.5%. In addition these resulted correlated well with the original application note 720003682en, (highlighted in Table 5). This performance and correlation with the original work demonstrates that artificial CSF containing BSA effectively eliminated NSB with performance comparable to those using 5% rat plasma as carrier protein and is a suitable alternate carrier protein for this assay.
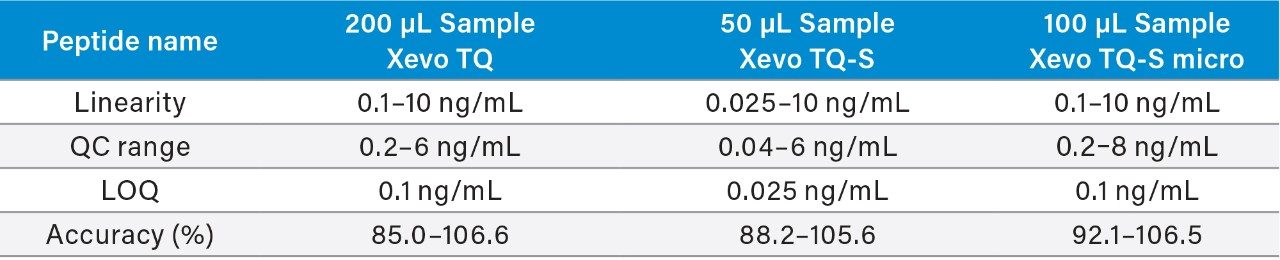
MRM transitions used for quantification are summarized in Table 1. Using the optimized SPE-LC-MS/MS method and the low cost TQ-S micro tandem quadrupole MS, highly robust and accurate quantification was achieved, demonstrating suitability for quantification of aβ peptides at the expected physiological levels found in hCSF. Xevo TQ-S micro MS quantification performance for the quantification of aβ peptides, compared to Xevo TQ and Xevo TQ-S MS Systems, is highlighted in Table 6. As expected, the Xevo TQ-S micro platform resulted in an analytical sensitivity decrease compared to the Xevo TQ-S, but similar to that described for the TQ. Using the optimized SPE-LC-MS/MS method with a 2-fold decrease in starting sample volume (100 vs. 200 μL), the low cost Xevo TQ-S micro Mass Spectrometer demonstrated highly robust and accurate quantification.
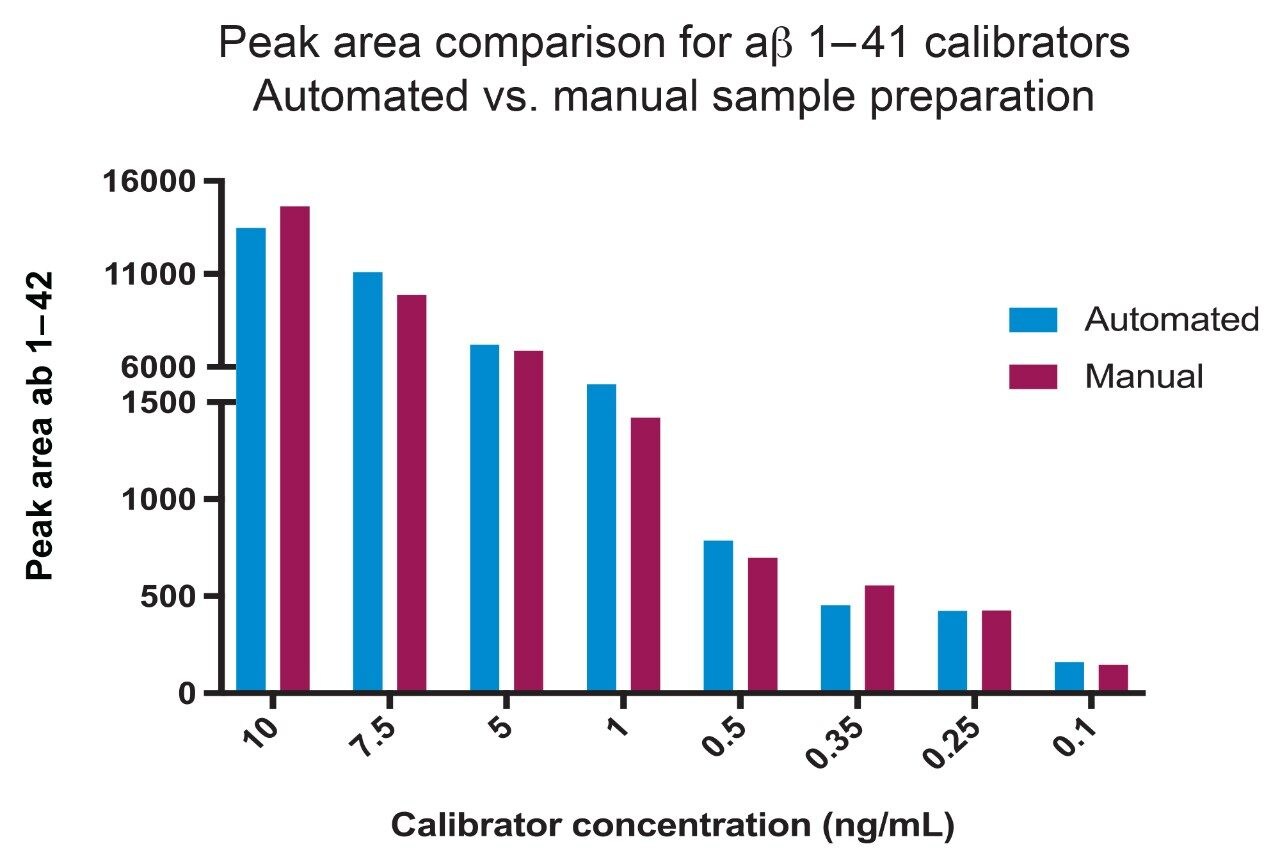
The introduction of the automated liquid handler has enabled enhanced speed, workflow standardization and hands-free highthroughput sample preparation, maximizing productivity and method robustness. Automation compatibility for the developed aβ SPE protocols (sample pre-treatment – Table 2, and SPE – Figure 1) was successfully demonstrated on the Tecan Freedom EVO 100/4. An example of automated vs. manual performance is demonstrated in Figure 5 comparing peak area’s for aβ 1–42. Results demonstrated no significant difference (p < 0.05) between the aβ 1–42 peak areas obtained for manually prepared samples vs. those samples prepared using a Tecan script. Additionally, there were no indications of loss of recovery in the automated sample preparation indicating that development of a validated automated pipetting script for the workflow is technically feasible.
720006517, February 2019