
In this application note, we explore the use of the NDR ratio in identifying ages of oils derived from three different sources, including the Beaufort Mackenzie Delta (Canada), the Danish North Sea and Greenland.
NDR ratios provide an extremely useful tool in assessing petroleum source age for oils from a range of different sources
Molecular biological markers, or biomarkers, are natural products that can be traced to a particular biological origin. They are structurally similar to, and are diagenetic alteration products of, specific natural products (compounds produced by living organisms) and have a wide variety of applications, e.g. in the study of ancient environments and in petroleum exploration. Specifically, biomarkers in an oil can reveal the age of the source rock, the environment of deposition as marine, lacustrine, fluvio-deltaic, or hypersaline, the lithology of the source rock (carbonate vs. shale), and the thermal maturity of the source rock during generation.
Examples of biological markers include acyclic isoprenoids and terpanes, steranes (tetracyclic triterpanes), and hopanes (pentacyclic triterpanes). The tetracyclic steranes originate from naturally occuring sterols. Sterols are widespread in animals and plants, where they are important in cell membranes. Diagenesis of sterols in sediments leads to sterenes and, ultimately, steranes. Not surprisingly, this is one of the largest classes of biomarkers.
Gas Chromatography-Mass Spectrometry (GC-MS) is the principal method used to detect and identify sterane isomers and other biological markers. Where the concentration of biomarkers of interest is relatively high, analysis of characteristic fragment ions in single ion recording (SIR) mode, e.g. using a single quadrupole instrument provides useful results. However, some oils have very low concentrations of the biomarkers of interest and abundant interfering compounds. Selective Ion Recording (SIR) of the 217 Da fragment ion is used to identify the steranes of interest. However, this type of analysis is prone to interferences from other families of compounds found in rock oil extracts including hopanes, methyl steranes and bicadinanes. In this case, tandem quadrupole instruments (GC-MS/MS) in Multiple Reaction Monitoring (MRM) mode provides a significant advantage over single quadrupoles, offering greater selectivity and sensitivity.

Asphaltenes were precipitated from the oil by addition of n-pentane (40x). The pentanesoluble fraction was concentrated and separated into saturated, aromatic, and polar compounds using MPLC.7
Saturate fractions were analysed by GC-MS/MS in MRM mode using the Waters Micromass Quattro micro GC Mass Spectrometer. The chromatography was completed using an Agilent 6890N gas chromatograph equipped with a splitless injector and fitted with a fused silica capillary column (30 m x 0.25 mm ID, 0.10 μm film thickness). 2 μL splitless injections were made; helium was used as carrier gas. The temperature program was: 70 °C isothermal for 2 mins, 30 °C/min ramp to 100 °C, 4 °C/min ramp to 308 °C, 308 °C isothermal for 8 minutes (total run time of 63 minutes). The temperature of the injector and the transfer line were both 250 °C. Argon was used as the collision gas. The typical sample concentration was 10 mg/mL isooctane. Data were acquired and processed using MassLynx Software.
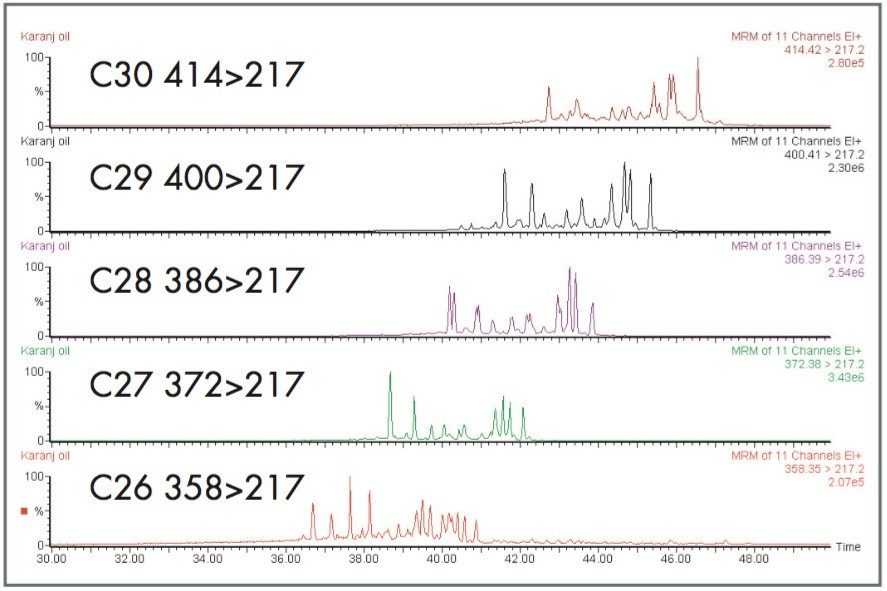
24-Norcholestanes are C26 steranes that show promise as indicators of geologic age and depositional environment.1 However, C26 steranes are generally present in oils and sediments in low concentrations giving GC-MS signals an order of magnitude lower than observed with the more common C27, C28, C29, and C30 steranes; therefore, GC-MS/MS analysis is required for greater specificity.
The high selectivity achievable by tandem quadrupole MS in MRM mode allows the biomarkers of interest to be clearly distinguished. In Figure 3, C26 to C30 sterane transitions are shown separately.

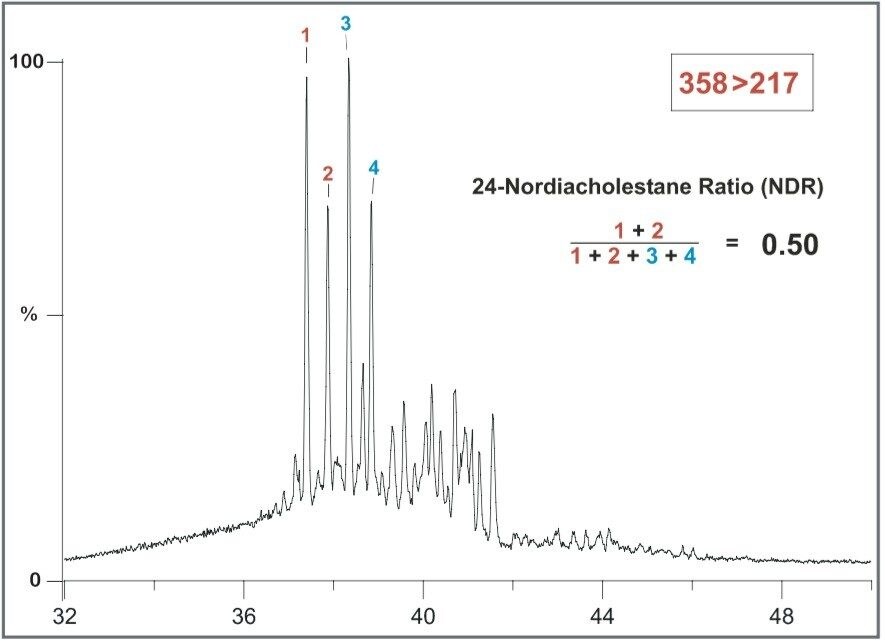
In 1998, Holba et al.1 observed that elevated 24-nordiacholestanes and 24-norcholestanes in Cretaceous or younger oils and sediments relative to their 27-norcholestane analogues may be related to geologic age. Two C26 sterane ratios, the 24 nordiacholestane ratio (NDR) and the 24-norcholestane ratio (NCR), may be defined respectively by equations (1) and (2) using peaks designated by the numbers in Figure 4.

The initial elevation of both the NCR and NDR ration occurs in the Jurassic4,5 (NDR>0.20, NCR>0.30). In the Cretaceous, a second and more significant increase in 24-norcholestane abundance occurs4,5,6 (NDR>0.25, NCR>0.40). There is strong circumstantial evidence that diatoms maybe the source of the 24-nor moiety. Indeed, in the Cretaceous, diatoms experienced a rapid expansion and species diversification.4,5,6
In this application note, we explore the use of the NDR ratio in identifying ages of oils derived from three different sources, including the Beaufort Mackenzie Delta (Canada), the Danish North Sea and Greenland.
The C26 steranes in oils from three different sources of known age have been identified by monitoring the MRM transition 358>217 and the 24-Nordiacholestane ratios calculated. The samples selected for this study include a Late Ordovician oil9 extract derived from Greenland (316082), a Late Jurassic oil extract from the North Sea (Mona-14,5) and an extract of Cretaceous Amauligak oil8 from Canada.
Figure 5 shows the C26 steranes in an extract of Amauligak oil from the Mackenzie Delta in Canada.8 The NDR ratio = 0.50, reflecting the relatively high abundance of the 24-Nordiacholestanes in this Cretaceous or younger oil.8
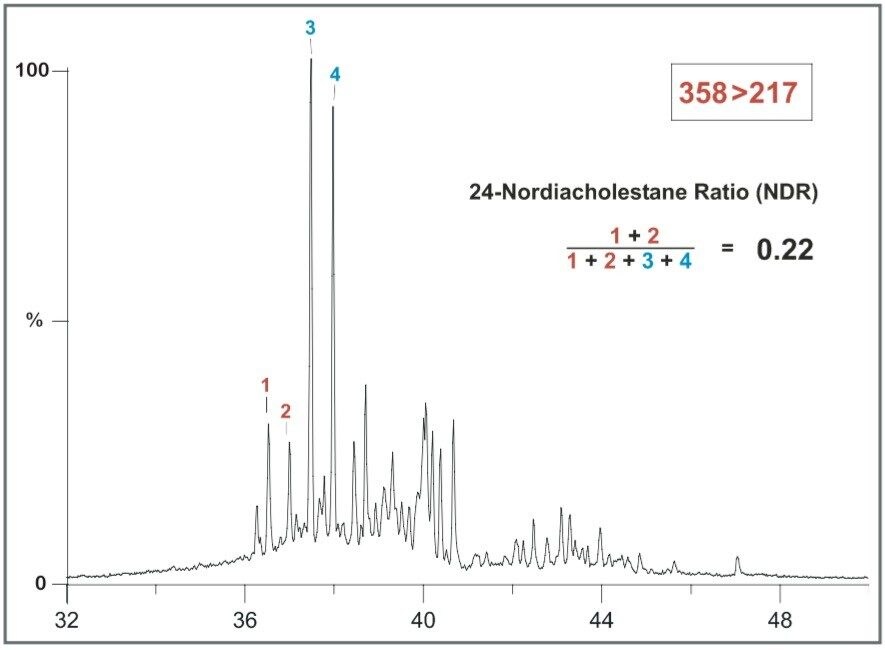
Mona-1 oil from the North Sea near Denmark4,5 has an NDR ratio of 0.22 (Figure 6), reflecting the Jurassic source. The 24-Nordiacholestanes (Peaks 1 and 2) are relatively low in abundance compared with the 27-Nor moieties (peaks 3 and 4).
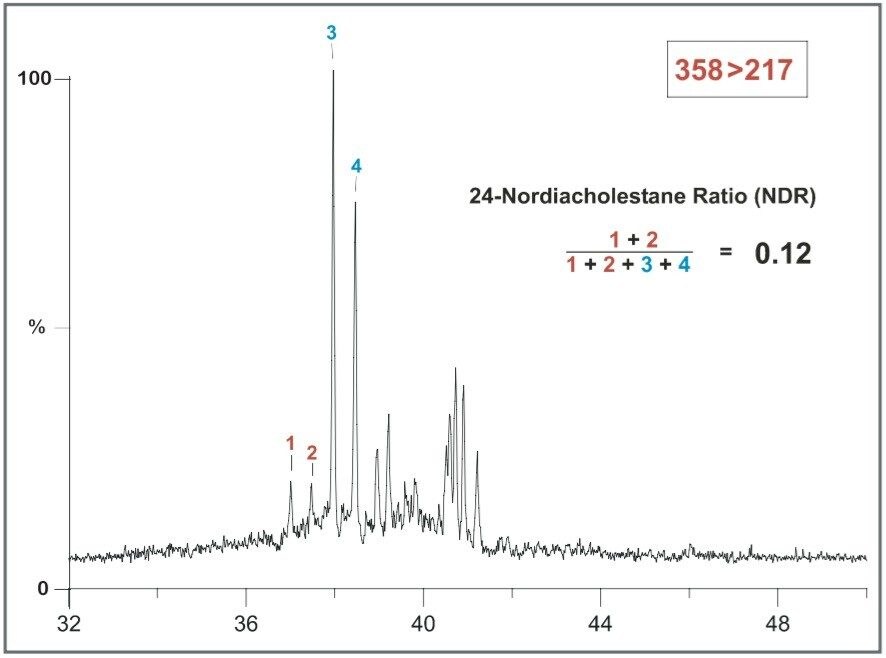
An NDR ratio of 0.12 is measured in the extract of the Late Ordovician source rock from North Greenland9 (Figure 7). The 24-Nordiacholestanes are very low in abundance in this sample.
720001085, February 2005