
Cyanobacteria (blue-green algae) are photosynthetic organisms found in both marine and freshwater environments. The occurrence of algal blooms has increased over time, and anthropogenic input of phosphorus and nitrogen into natural water bodies are contributors to this increase in toxic algal blooms worldwide. Many species of cyanobacteria produce secondary metabolites, some of which are toxic to higher organisms. Microcystins are a group of non-ribosomally synthesized cyclic heptapeptides, of which microcystin-LR (MC-LR) is the most common.
Microcystins can be introduced to tissues of organisms through the diet or by ingestion of contaminated water. Microcystins are readily water-soluble so once ingested they accumulate in the liver via the bile acid transport system. The risk to human health due to the increasing presence of these toxins is concerning.
The World Health Organization (WHO) provisional guideline value for MC-LR of 1 μg/L, which was deemed protective of total microcystins, has been adopted as a basis for national standards or guideline values for drinking water in many countries.
Detection of microcystins is a challenging problem for water testing laboratories. Many laboratories have turned to liquid chromatography- tandem mass spectrometry (LC-MS/MS) for the concurrent analysis of a range of cyanotoxins. This application note describes a method for the analysis of drinking and surface waters for 10 well-known cyanotoxins;cylindrospermopsin (CYL), anatoxin-a (ANA) and microcystins nodularin (NOD), microcystin-LR (MC-LR), MC-DeRR, MC-RR, MC-YR, MC-LY, MC-LW, and MC-LF. The method uses direct injection of 50 μL of water sample with the ACQUITY UPLC I-Class System coupled to Xevo TQ-S.
This application note describes a UPLC-MS/MS method for the analysis of 10 cyanotoxins, including important microcystins, using standard addition, without the need for extraction at concentrations well below the WHO provisional guideline of 1 μg/L.
Cyanobacteria (blue-green algae) are photosynthetic organisms found in both marine and freshwater environments. The occurrence of algal blooms has increased over time, and anthropogenic input of phosphorus and nitrogen into natural water bodies are contributors to this increase in toxic algal blooms worldwide. Many species of cyanobacteria produce secondary metabolites, some of which are toxic to higher organisms. Microcystins are a group of non-ribosomally synthesized cyclic heptapeptides, of which microcystin-LR (MC-LR) is the most common (Figure 1). About 90 microcystin variants have been identified, including a number of microcystin-LR homologues, some of which have similar toxicity to MC-LR. Their hepatotoxicity may cause serious damage to the liver. Microcystins can be introduced to tissues of organisms through the diet or by ingestion of contaminated water. Microcystins are readily water-soluble so once ingested they accumulate in the liver via the bile acid transport system. The risk to human health due to the increasing presence of these toxins is of concern.1,2
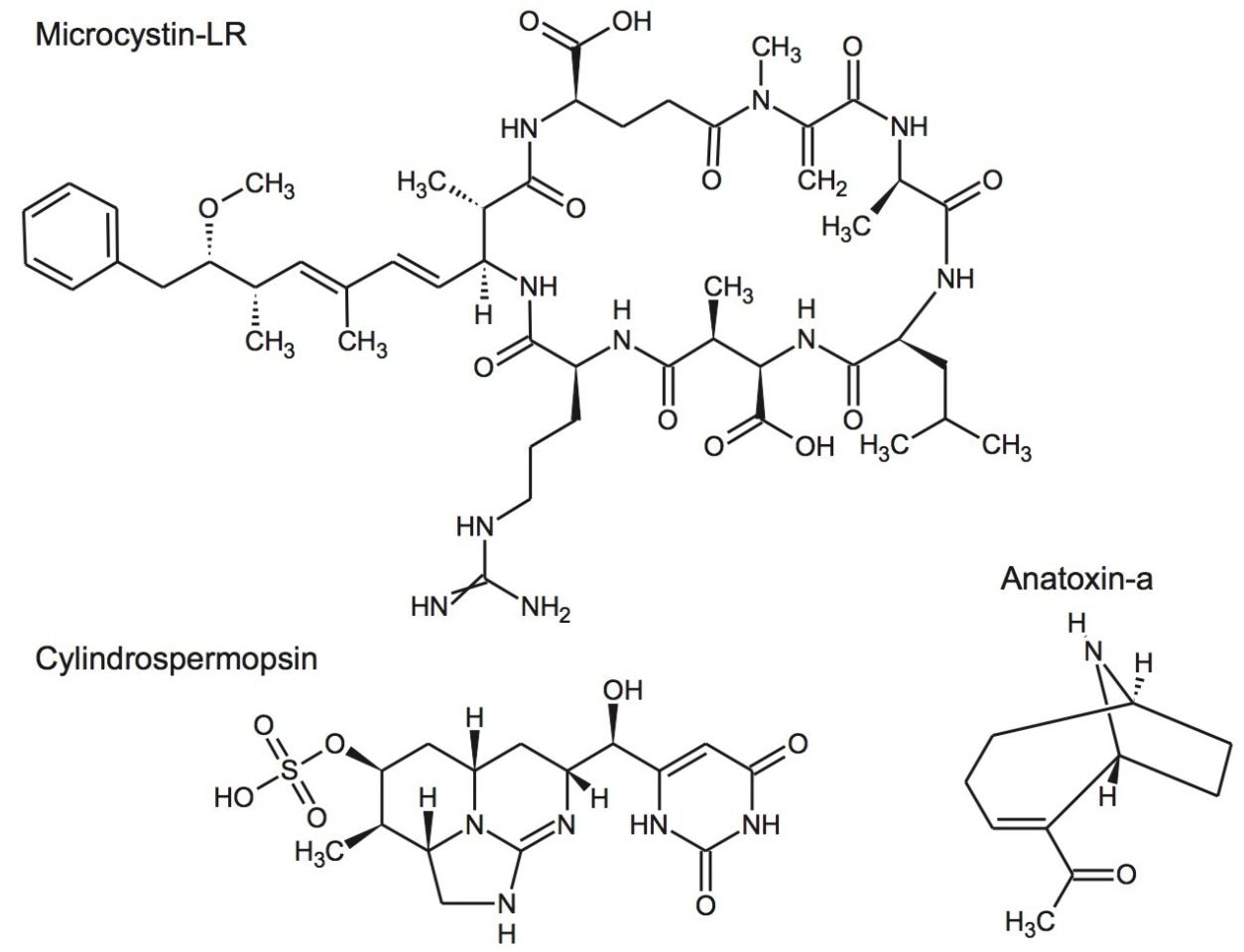
The World Health Organization (WHO) provisional guideline value for MC-LR of 1 µg/L, which was deemed protective of total microcystins, has been adopted as a basis for national standards or guideline values for drinking water in many countries.3 Some countries set limits for microcystins in water bodies used for recreation and these tend to be at higher concentrations (typically between 10 and 100 µg/L). Other cyanotoxins, such as cylindrospermopsin and anatoxin-a (Figure 1), are regulated in some countries by the use of guidance values and health alert limits.
Detection of microcystins is a challenging problem for water testing laboratories. Historically, antibody-based enzyme-linked immunosorbent assays (ELISAs) have been used to monitor microcystin concentrations in water and in extracted bloom samples. However, ELISAs do not differentiate between free microcystin and its conjugates or between different congeners of microcystins. Hence, many analyses conducted using ELISA are susceptible to generation of false positive results for microcystins. Other cyanotoxins, anatoxins and cylindrospermopsin, are structurally and biologically different to MC-LR, so would require separate ELISA tests. In response, many laboratories have turned to liquid chromatography- tandem mass spectrometry (LC-MS/MS) for the concurrent analysis of a range of cyanotoxins.
This application note describes a method for the analysis of drinking and surface waters for 10 well-known cyanotoxins; cylindrospermopsin (CYL), anatoxin-a (ANA) and microcystins nodularin (NOD), microcystin-LR (MC-LR), MC-DeRR, MC-RR, MC-YR, MC-LY, MC-LW, and MC-LF. The method uses direct injection of 50 µL of water sample with Waters® ACQUITY UPLC I-Class System coupled to Xevo TQ-S.
Drinking and surface water samples were injected as supplied without any further preparation.
|
UPLC system: |
ACQUITY UPLC I-Class with FTN autosampler |
|
Column: |
ACQUITY UPLC BEH C18 1.7 μm, 2.1 x 100 mm |
|
Mobile phase A: |
0.1% formic acid (aq.) |
|
Mobile phase B: |
acetonitrile |
|
Flow rate: |
0.4 mL/min |
|
Injection volume: |
50 μL |
|
Column temp.: |
50 °C |
|
Sample temp.: |
10 °C |
|
Run time: |
7.5 min |
|
Time (min) |
%A |
%B |
Curve |
|---|---|---|---|
|
0 |
100 |
0 |
– |
|
0.75 |
100 |
0 |
6 |
|
5 |
20 |
80 |
6 |
|
6 |
0 |
100 |
1 |
|
7.5 |
100 |
0 |
1 |
|
MS system: |
Xevo TQ-S |
|
Source: |
Electrospray |
|
Ionization mode: |
ESI+ |
|
Capillary voltage: |
3.0 kV |
|
Desolvation temp.: |
600 °C |
|
Desolvation gas flow: |
1000 L/Hr |
|
Source temp.: |
150 °C |
|
Cone gas flow: |
150 L/Hr |
|
Collision gas flow: |
0.15 mL/min |
|
Nebulizer gas pressure: |
7 Bar |

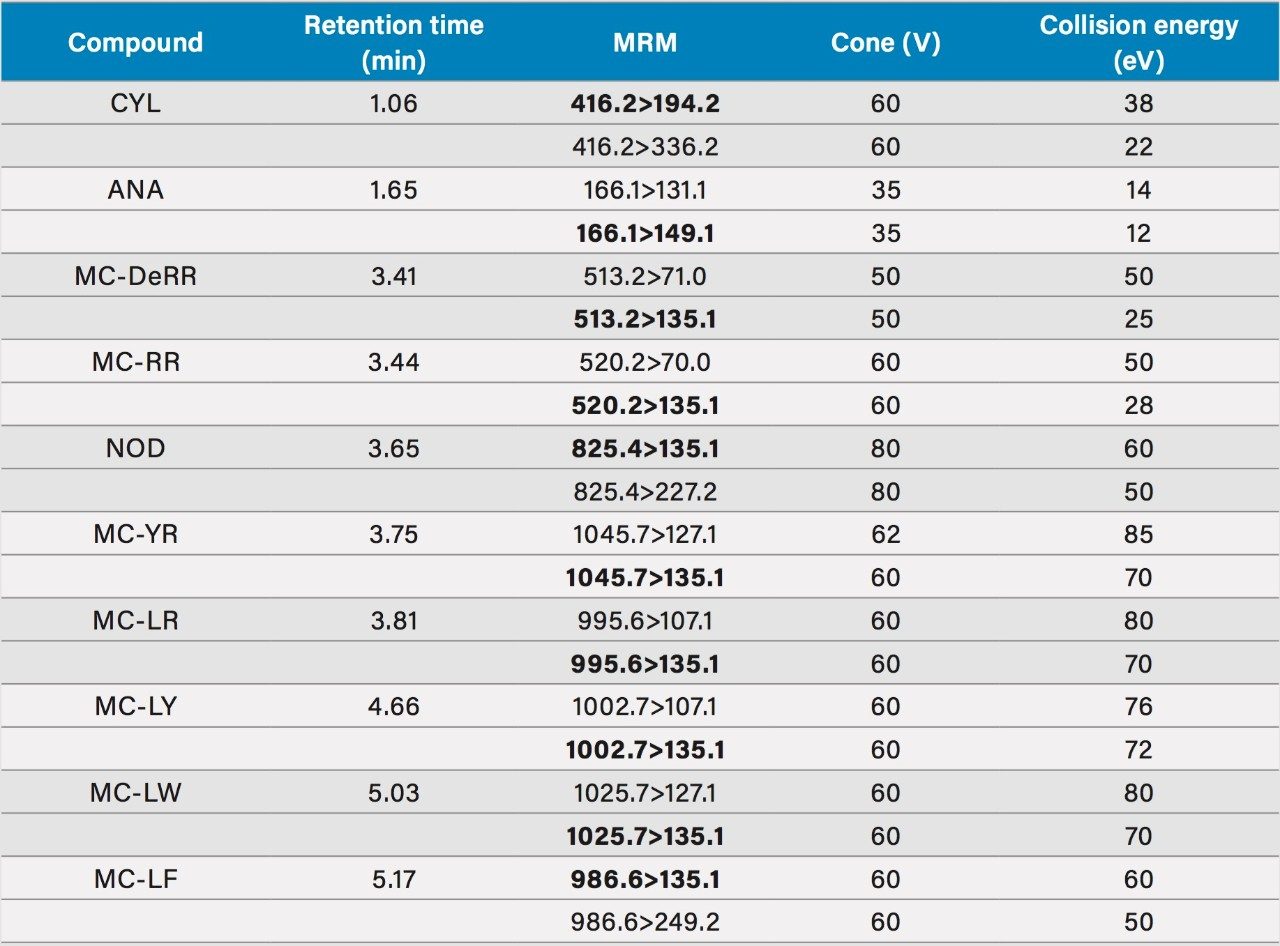
Data were was acquired using MassLynx MS Software and processed using TargetLynx XS Application Manager. The selection of MRM transitions and optimization of critical parameters was performed by infusion of individual solutions of all the analytes and evaluation of the data by IntelliStart Software to automatically create acquisition and processing methods. Two MRM transition were used, unless otherwise stated. Table 1 summarizes conditions for all MRM transitions including the retention times. The optimum dwell time was set automatically using the Autodwell function based upon 4s wide peaks and 16 data points per peak.
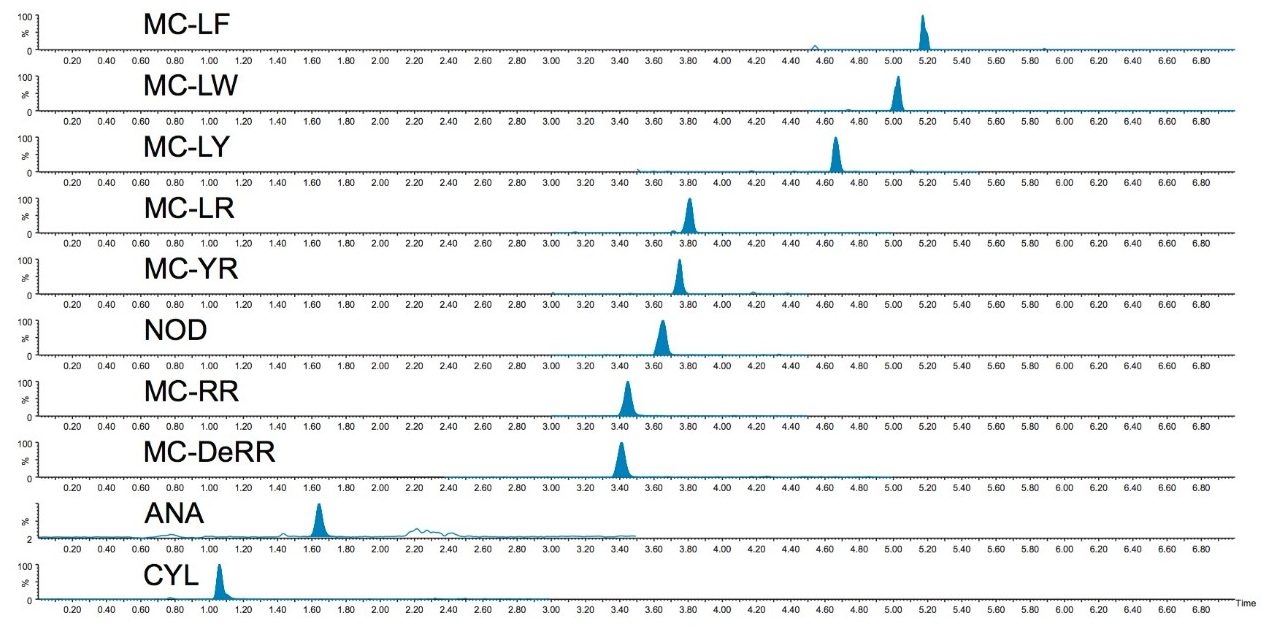
Excellent sensitivity and selectivity was demonstrated by the response for each analyte peaks detected from the analysis of water (as shown in Figure 2). No interfering compounds were detected at the retention times of the analytes in all the tested blank samples. All compounds can be easily detected at the 25 ng/L level, well below the WHO provisional guideline value for MC-LR of 1 μg/L and demonstrates the method would be suitable for determination at the lowest of the concentration required to meet guidance/action levels for drinking water adopted by four states in the U.S. (100 ng/L), without the need for on-line enrichment.
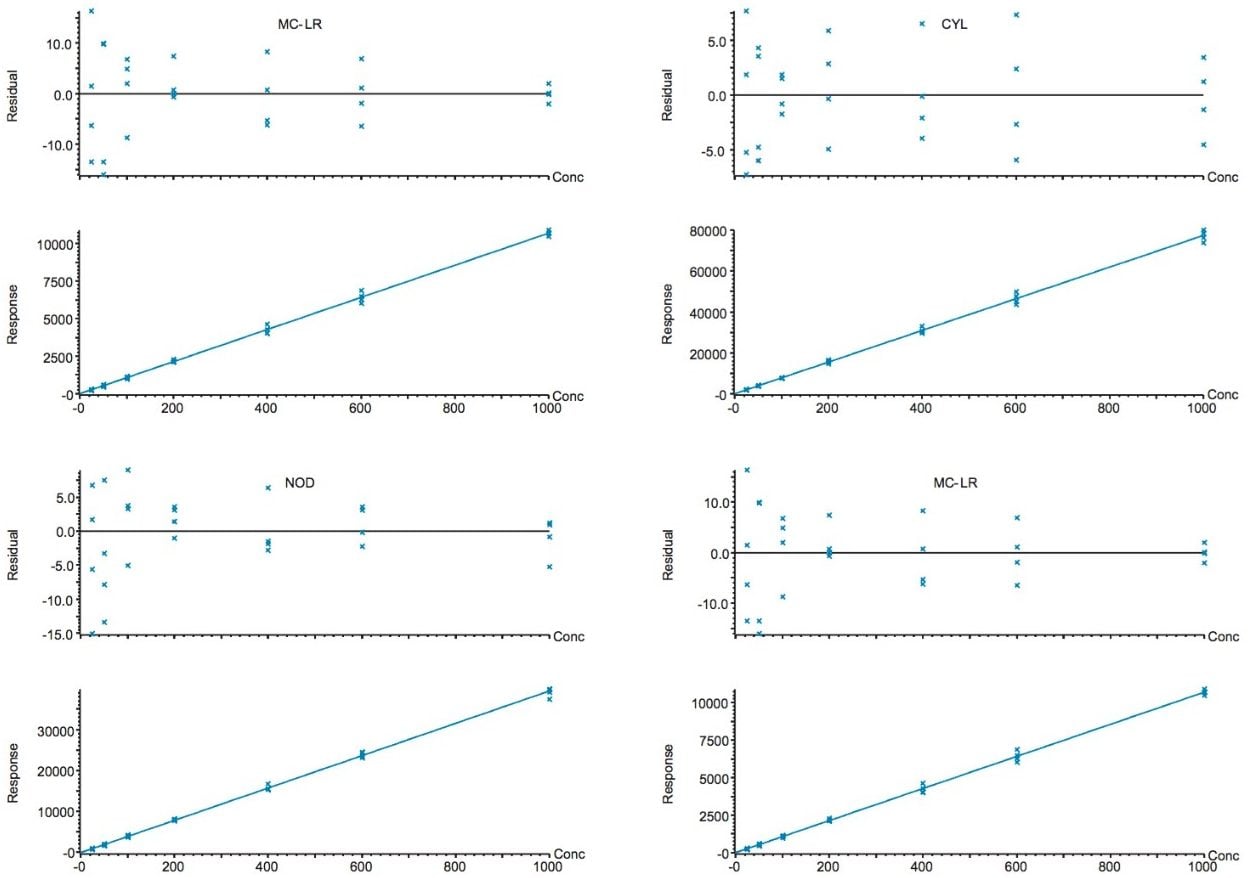
Linearity in drinking and surface water was evaluated over the concentration range 10 to 1000 ng/L for each of the analytes. Figure 3 shows a selection of calibration graphs, each an overlay of 2 calibration curves, bracketing the injections of the spiked water samples. The coefficients of determination were satisfactory (r2>0.99) and the residuals <20%.
Short-term repeatability was evaluated from replicate injections of drinking water (DW) and surface water (SW), previously spiked at 100 ng/L. Precision, as measured from the peak areas of replicate (n=5) injections varied from 0.5 to 5.0% RSD.
The method's accuracy was evaluated by measuring the concentration of eight of the cyanotoxins in drinking water and surface water samples, spiked at 100, 200, and 400 ng/L five times, using a bracketed calibration series of standards prepared in drinking water. The accuracy of the concentrations measured in both sets of water samples was poor. Measured values were consistently underestimated in drinking water and highly variable in the surface water, indicating a presence of ion suppression due to matrix effects. Such matrix effects can be compensated for by the use of internal standards, but since stable isotope analogues for microcystins are not widely available, and those that do exist are very expensive, standard addition offers an alternative means of quantification.
The standard addition methodology was used for the quantification of cyanotoxins in recreational lake water. Standard addition is a type of quantitative analysis whereby known amounts of standard are added directly to test portions of each water sample to be analyzed. This can be done in an automated manner using the Auto Addition feature in the ACQUITY UPLC Sample Manager driver, which provides the option to add known amounts of cyanotoxin standard solution to vials of the sample. Data from the analysis of the sample and test portions of the sample spiked with known amounts of standard solution was processed to provide a concentration of any cyanotoxins in the sample.
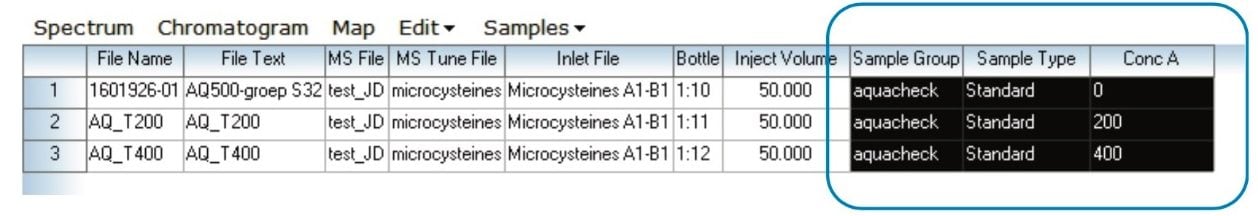
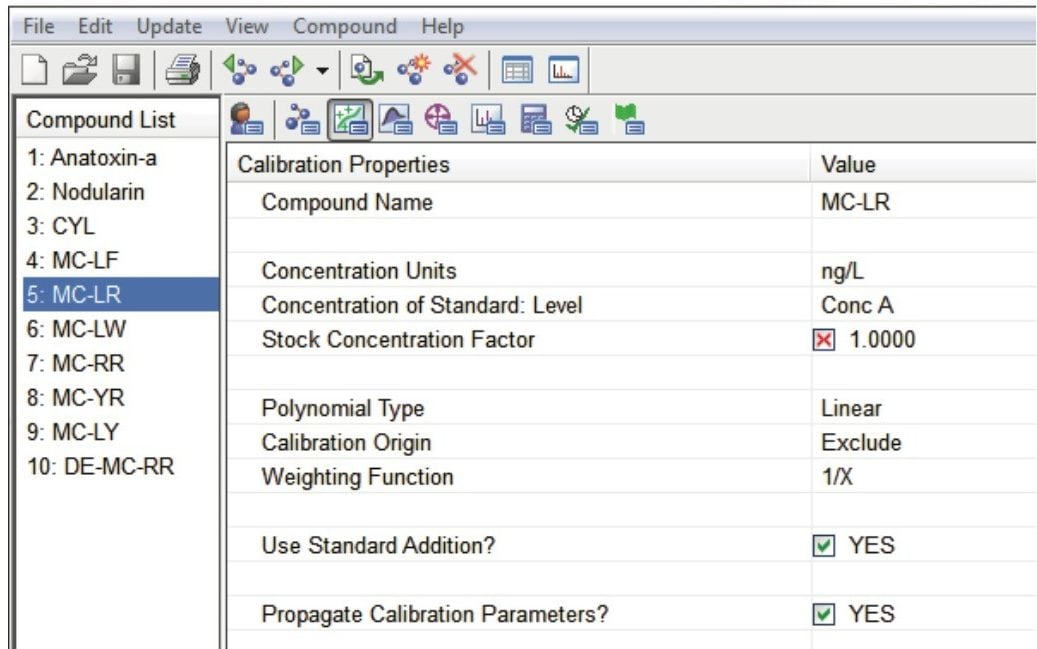
The standard addition calculations were carried out automatically in the TargetLynx XS Application Manager. Figures 4 and 5 show the selection of key parameters in the MassLynx sample list and in the TargetLynx XS method. In the sample list, select Standard for sample type of all test portions of the sample. Add the spiked concentration in the concentration column. Finally assign a sample group for each set of samples. In the TargetLynx XS processing method, check the Use Standard Addition? option.
As a result of the processing of the samples, a TargetLynx XS dataset is created, displaying the calculated concentration in the summary table, as well as the standard addition calibration curve (Figure 6). The concentration of MC-LR in the PT sample, calculated by standard addition, was 724 ng/L, which was in good agreement with the assigned value of 720 ng/L.
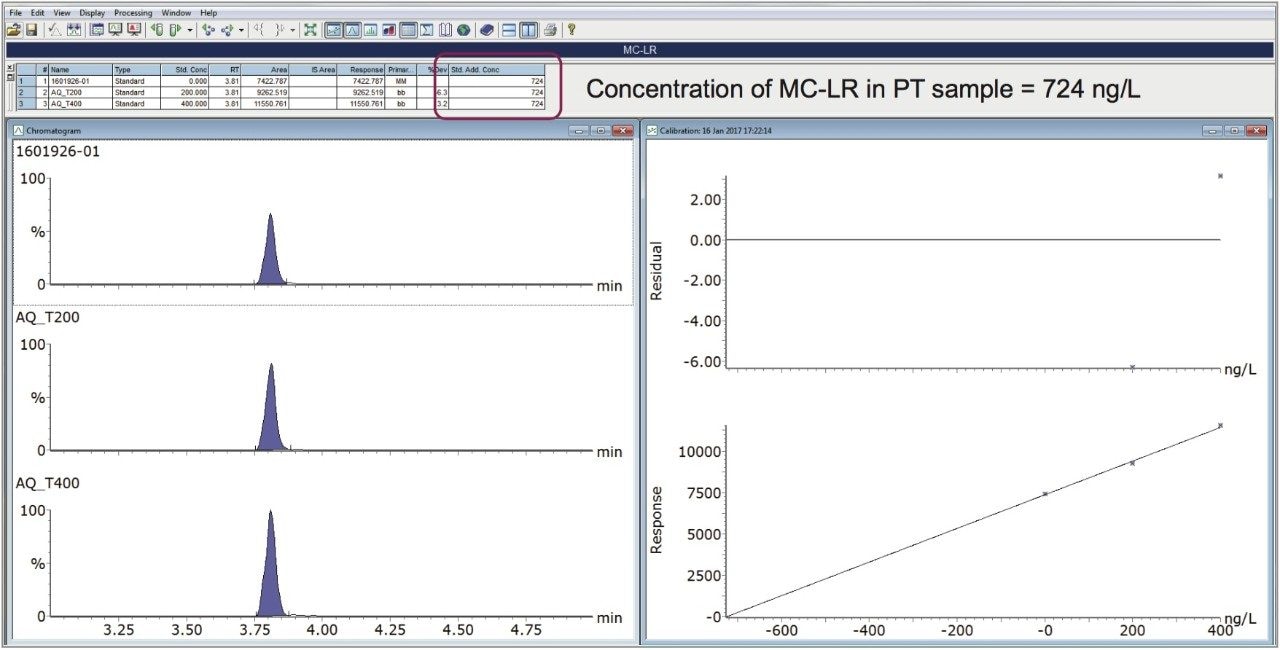
This application note describes the performance of a method for the analysis of 10 cyanotoxins, including important microcystins, by UPLC-MS/MS, using direct injection on an ACQUITY UPLC I-Class System coupled to Xevo TQ-S. This method provides fast and reliable quantitation of the presence of cyanotoxins in water samples using standard addition, without the need for extraction, at concentrations well below the WHO provisional guideline value for MC-LR of 1 µg/L. The response was stable for up to 15 hours of analysis during the validation study, providing good repeatability (RSD <5%). Calibration characteristics, linearity and residuals, were excellent over the concentration range studied. To overcome matrix effects, in the absence of internal standards, standard addition provided an accurate means for quantitation of microcystins in lake water, as demonstrated by successful determination of MC-LR in an independent Aquacheck proficiency test lake water sample.
Waters gratefully acknowledges the Watergroep, Heverlee, Belgium.
720005939, April 2017