For forensic toxicology use only.
This is an Application Brief and does not contain a detailed Experimental section.
The purpose of this study was to evaluate the ACQUITY UPLC H-Class/ACQUITY QDa System as an alternate technique to potentially replace two commonly applied immunoassays by developing a single, fast analytical method which incorporates a simple sample preparation procedure. Selectivity is achieved by monitoring retention time, precursor and product ion(s), and ion ratios. The method also comprised an analytical range which extended to 2000 ng/mL, offering the user an ability to quantify.
The ACQUITY QDa Mass Detector has demonstrated great promise as an alternative technique to immunoassay.
Amphetamines (methamphetamine and amphetamine) are among the most commonly abused illicit substances in the world. In 2016, it was estimated that around 35 million adults worldwide used amphetamines.¹ The region with the highest annual use was North America at approximately 2% of the population. In the last few years however, there also appears to be a marked increase in consumption within other geographies, particularly East and Southeast Asia, where the amphetamines have been identified as one of the most worrying threats of drug use. Moreover, a rise in the use of Ecstasy (3,4 methylenedioxymethamphetamine; MDMA) in West Asia has been noted, though it is estimated that less than half of the tablets purported to be Ecstasy, truly contain MDMA, but are commonly found to contain methamphetamine, amphetamine, or ketamine.²
Forensic laboratories often utilize immunoassay for routine urine analysis. While the technique is rapid and simple, there can be some disadvantages, often associated with poor selectivity for example, false positive results for amphetamine or ketamine have been reported for a variety of substances.3-5 As most positive immunoassay results require a confirmatory test (such as GC or LC-MS), the consequence of false positive identifications, as a result of poor assay selectivity, can significantly compromise laboratory efficiency.
The purpose of this study was to evaluate the UPLC-QDa system as an alternate analytical technique for the analysis of two commonly abused drug classes. This technique uses chromatographic separation coupled with a simple mass detector to address the issues of cross-reactivity and laboratory inefficiencies associated with immunoassay.

Certified reference standards (1 mg/mL) and their corresponding deuterated internal standards (ISTDs) (100 μg/mL) were purchased from Sigma-Aldrich (Poole, UK; Table 1). A commercial quality control (QC) urine sample (Medidrug WDT Confirm U, -25% cutoff; p/n: 27UQ01KE) containing amphetamine, methamphetamine, and ketamine at 150 ng/mL was purchased from Medichem (Germany). Authentic drug-free urine samples were collected from volunteers and pooled.
To evaluate analytical selectivity and linearity, a series of calibrators ranging from 40 to 2000 ng/mL, were prepared by spiking mixed reference standards into the pooled drug-free urine. A series of in-house QCs (75, 150, 600, and 1600 ng/mL) were prepared from a separate batch of reference material in pooled urine.
Prior to analysis, all samples and QCs were diluted 5-fold in an aqueous solution containing the ISTDs at a concentration of 500 ng/mL.
Chromatographic separation was achieved using a fast 3 min gradient elution on an ACQUITY UPLC BEH C18, 1.7 μm, 2.1 × 50 mm Column coupled with an ACQUITY UPLC BEH C18, 1.7 μm, 2.1 x 5 mm VanGuard Pre-Column on the ACQUITY UPLC H-Class System. Data were acquired with the ACQUITY QDa Mass Detector in positive ionization mode applying selected ion recording (SIR). In-source collision-induced dissociation (CID) was applied at specific cone voltages to generate at least one product ion.6,7
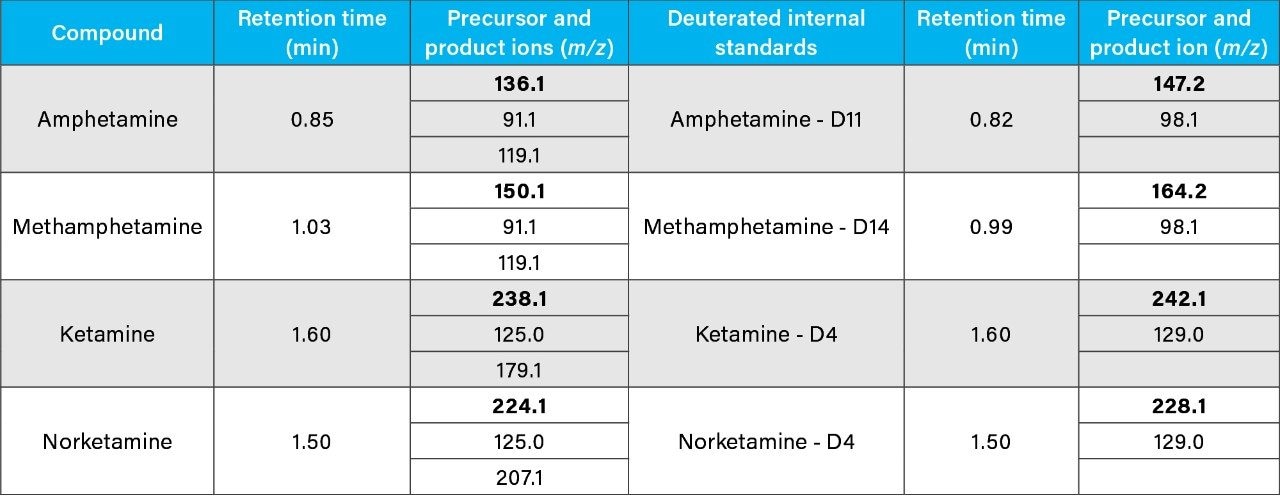
Data were acquired using MassLynx Software and processed using the TargetLynx Application Manager. The following criteria were used for a positive identification: presence of the precursor ion together with the product ion(s) at the expected retention time (±0.2 min) and with an ion ratio of ±20% of the expected value. Reference ion ratios were based on the ratio of peak-area response of the precursor to the response of the associated product ion(s), and were calculated from the average obtained across the four QC concentrations.
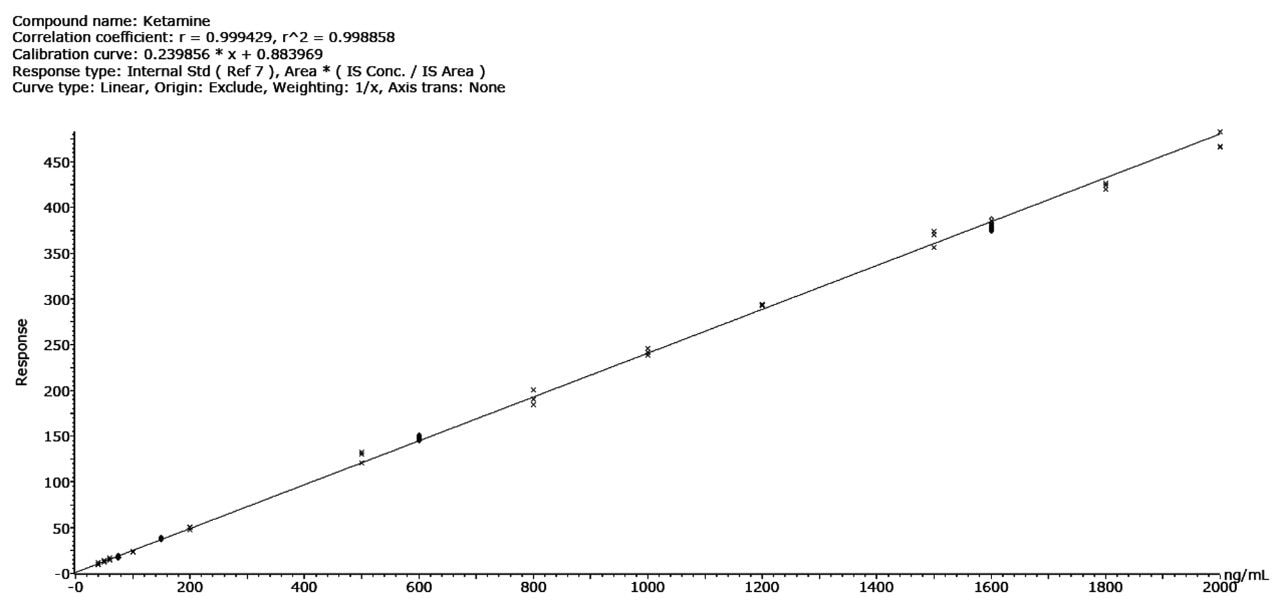
All compounds demonstrated good linearity across the concentration range investigated (40–2000 ng/mL); Figure 2 shows an example of the response for ketamine.
The analysis of the commercial urine QC (WDT -25%) showed the successful identification and the calculated concentrations for amphetamine, methamphetamine, and ketamine were within 12% of expected concentrations.
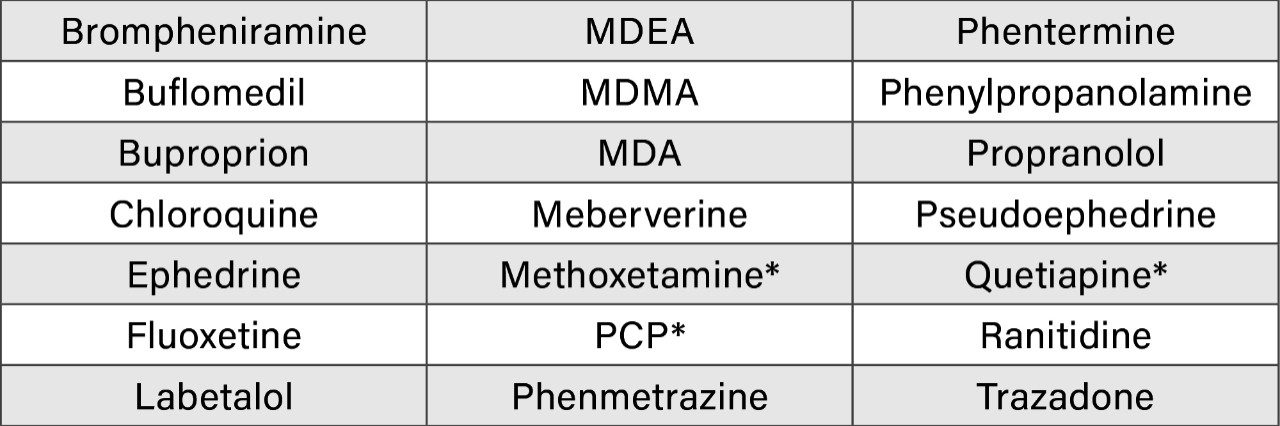
To assess the effect of some common analytes that are known to interfere with the amphetamine or ketamine immunoassays, 21 substances (Table 2) were selected for investigation. Blank urine was spiked individually with each of the 21 standards at a concentration of 10,000 ng/mL. None of the investigated analytes resulted in a false positive result for amphetamine, methamphetamine, ketamine, and norketamine. In addition, the potential interference was investigated by spiking a QC urine (600 ng/mL) with the same analytes – the results showed no interference in terms of quantitative data.
A selection of authentic urine samples, previously characterized using an established high resolution mass spectrometry screening technique based on the Xevo G2-XS QTof, were tested.⁸ The results showed good agreement between the two approaches.
Although LC-MS/MS is now established for illicit drug screening and quantification, in forensic toxicology laboratories worldwide, this study shows that for some applications, a single quadrupole-based detector can provide sufficient analytical sensitivity and dynamic range. The ACQUITY QDa Mass Detector has demonstrated great promise as an alternative technique to immunoassay.
Drug testing facilities face a perpetual struggle to provide rapid and accurate results. The purpose of this study was to evaluate the ACQUITY UPLC H-Class/ACQUITY QDa System as an alternate technique to potentially replace two commonly applied immunoassays by developing a single, fast analytical method which incorporates a simple sample preparation procedure. Selectivity is achieved by monitoring retention time, precursor and product ion(s), and ion ratios. The method also comprised an analytical range which extended to 2000 ng/mL, offering the user an ability to quantify.
720006595, June 2019