For research use only. Not for use in diagnostic procedures.
Here we present a targeted UPLC-MS/MS methodology for the semi-quantitative profiling of bioactive oxylipins (oxidized fatty acids) in plasma/serum and cell culture media. The combination of mixed mode solid-phase extraction (Oasis MAX µElution SPE) and UPLC-MS/MS provides a broad ranging analysis of oxylipins for use in targeted workflows. Retention times and MRM transitions for 92 oxylipins are detailed for routine, targeted analysis of samples in biomedical research studies.
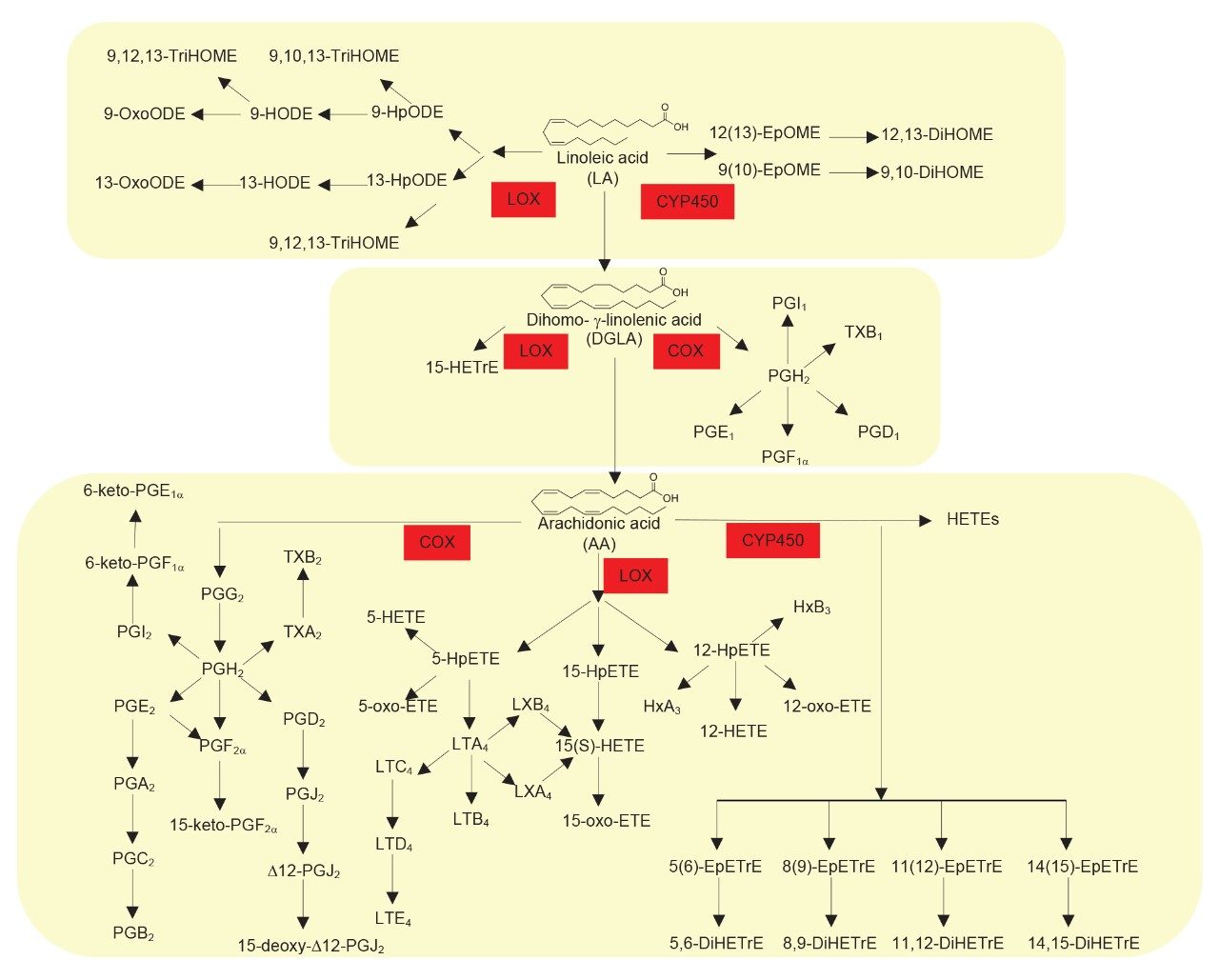
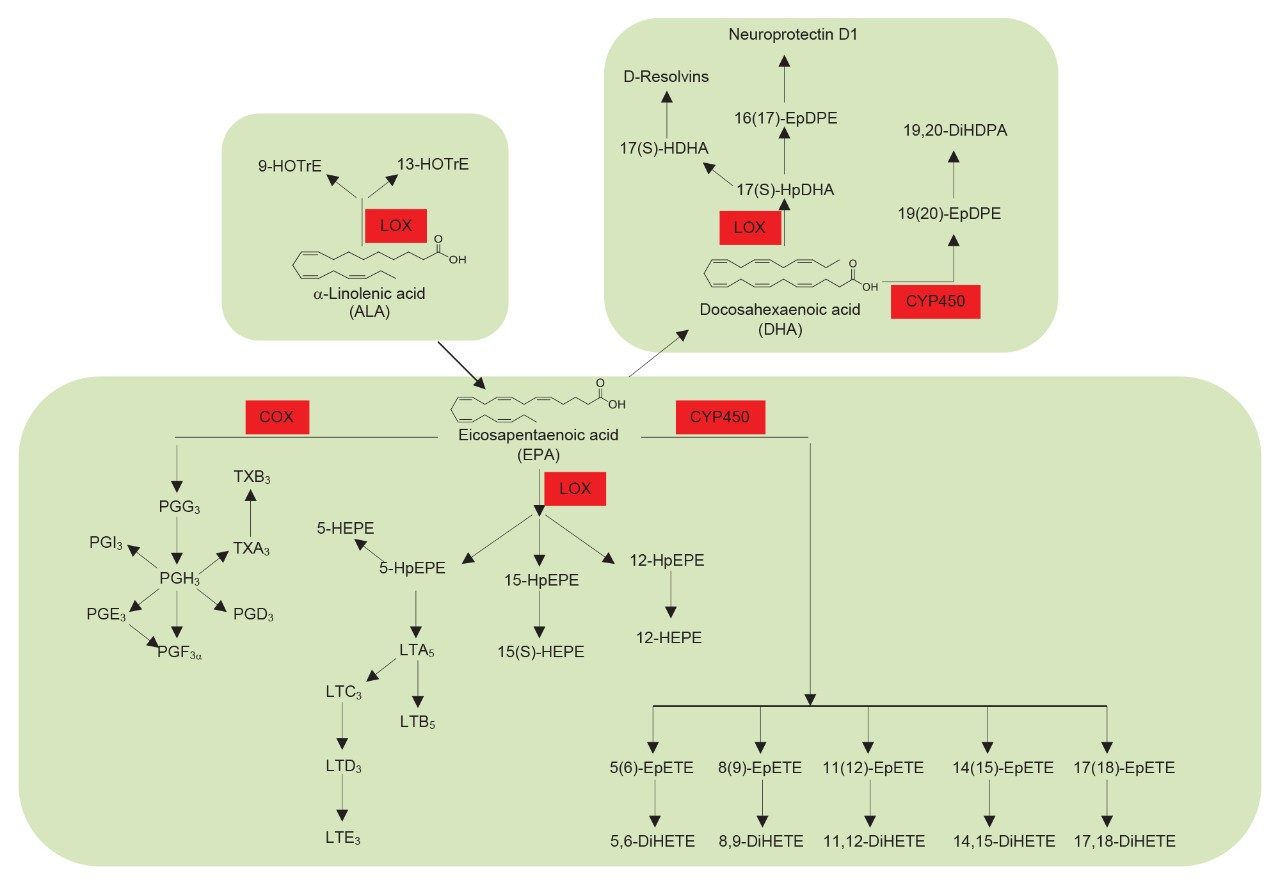
Oxylipins are important bioactive lipids that play prominent roles in many key biological processes, most notably in instigation and resolution of inflammation. As such, the semi-quantitative profiling of a wide range of oxylipins in plasma/serum and cell culture samples would be advantageous for use in biomedical research studies. Oxylipins are produced by enzymatic and non-enzymatic oxygenation of omega-6 polyunsaturated fatty acids (linoleic acid, adrenic acid, and arachidonic acid) and omega-3 polyunsaturated fatty acids – α-linolenic acid, eicosapentaenoic acid and docosahexaenoic acid – see Figures 1 and 2. The major metabolic pathways involved in the generation of this class of compounds are the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) pathways. These pathways are important drug targets for multiple diseases and therefore a semi-quantitative profiling methodology is highly relevant in drug discovery. The semi-quantitative analysis of oxylipins is however very challenging, due to their low endogenous concentration and their limited stability within samples. In addition, the same fatty acid precursor can react at different positions on the molecule, leading to multiple isomeric species. An analytically specific and sensitive methodology is therefore required. Analysis of oxylipins has previously been performed using radiometric and enzymatic immunoassays, but these methods often lack selectivity and target a limited number of compounds. Gas chromatography (GC–MS) methods have also been used, but these methods require complex procedures involving derivatization. Here we demonstrate a targeted UPLC-MS/MS method for the profiling of 92 oxylipins in plasma/serum and cell culture samples.
UPLC separation was performed with an ACQUITY UPLC I-Class System equipped with an ACQUITY Premier BEH C18 (2.1 x 150 mm) analytical column maintained at 35 °C. A 3 μL aliquot of the sample was injected onto the column and eluted under reversed–phase gradient conditions where mobile phase A comprised 0.01% formic acid(aq) and mobile phase B comprised 0.01% formic acid in acetonitrile. Oxylipins were eluted from the column and separated using a 3-stage linear gradient. An initial stage of 25–28% mobile phase B over 4 minutes was followed by a second phase of 28–32% mobile phase B over 8 minutes. Finally, a third stage of 14 minutes from 32–95% mobile phase B was followed by a 2-minute column flush and then re-equilibration back to starting conditions.
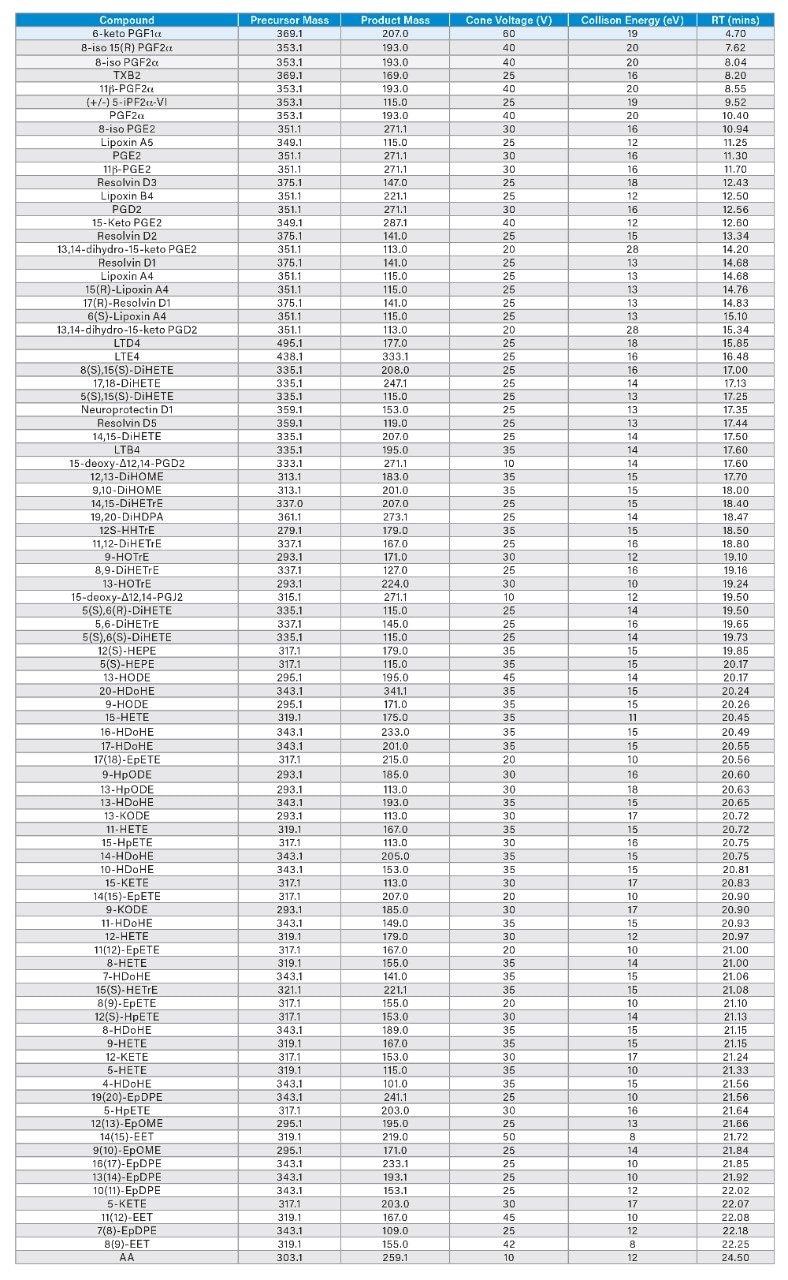
Oxylipins were detected using Multiple Reaction Monitoring (MRM) analyses on a Xevo TQ-XS Mass Spectrometer. All experiments were performed in negative electrospray ionization (ESI-) mode. The ion source temperature and capillary voltage were kept constant and set to 150 °C and 2.0 kV, respectively. The cone gas flow rate was 50 L/hr and desolvation temperature was 650 °C. The MRM transitions employed are detailed in Table 1.
Method information was imported onto the LC-MS system using the Quanpedia functionality within MassLynx. This extendable and searchable database produces LC and MS methods as well as processing methods for use in TargetLynx for compound quantification.
|
LC system: |
ACQUITY UPLC I-Class |
|
Detection: |
Xevo TQ-XS |
|
Column(s): |
ACQUITY Premier BEH C18 (2.1 x 150 mm) |
|
Column temp.: |
35 °C |
|
Sample temp.: |
6 °C |
|
Injection volume: |
3 µL |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
0.01% formic acid (aq) |
|
Mobile phase B: |
0.01% formic acid in acetonitrile |
|
Gradient: |
0–4 minutes 25–28% B 4–12 minutes 28–32% B 12–26 minutes 32–95% B |
|
MS system: |
Xevo TQ-XS |
|
Ionization mode: |
ESI negative |
|
Capillary voltage: |
2.0 kV |
The focus of this work was to demonstrate a sensitive and specific UPLC-MS/MS method for profiling bioactive oxylipins in plasma, serum, and cell culture samples. Oxylipins are present at very low levels in biological samples and good sample preparation is very important for successful analysis. In order to remove as many non-relevant matrix components as possible–and to maximize sensitivity for the low abundance analytes – we used an anion exchange, mixed mode solid-phase extraction (Oasis MAX SPE) prior to UPLC-MS/MS analysis. This type of SPE specifically targets and extracts acids from the matrix resulting in much cleaner samples than a standard SPE method.
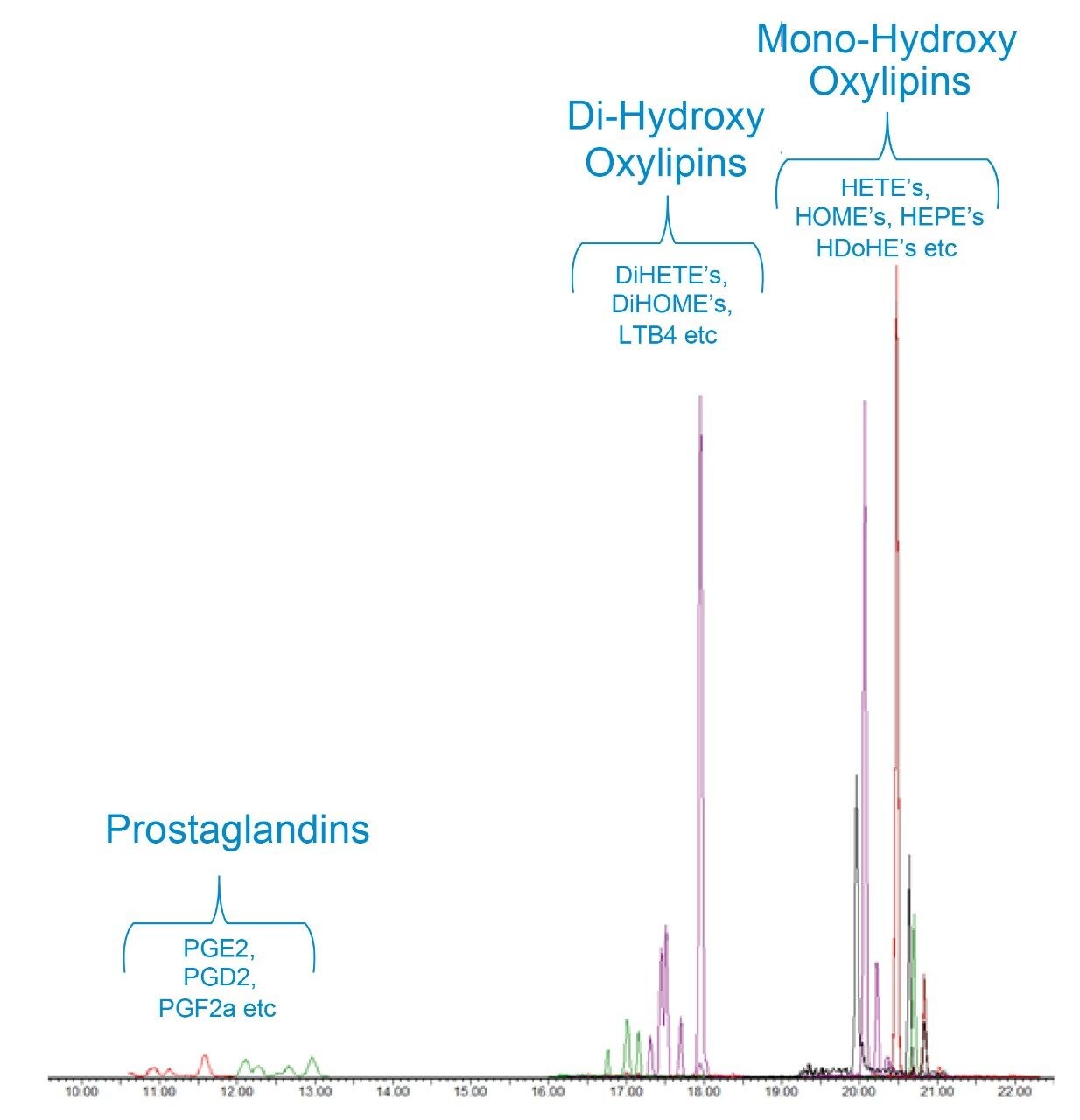
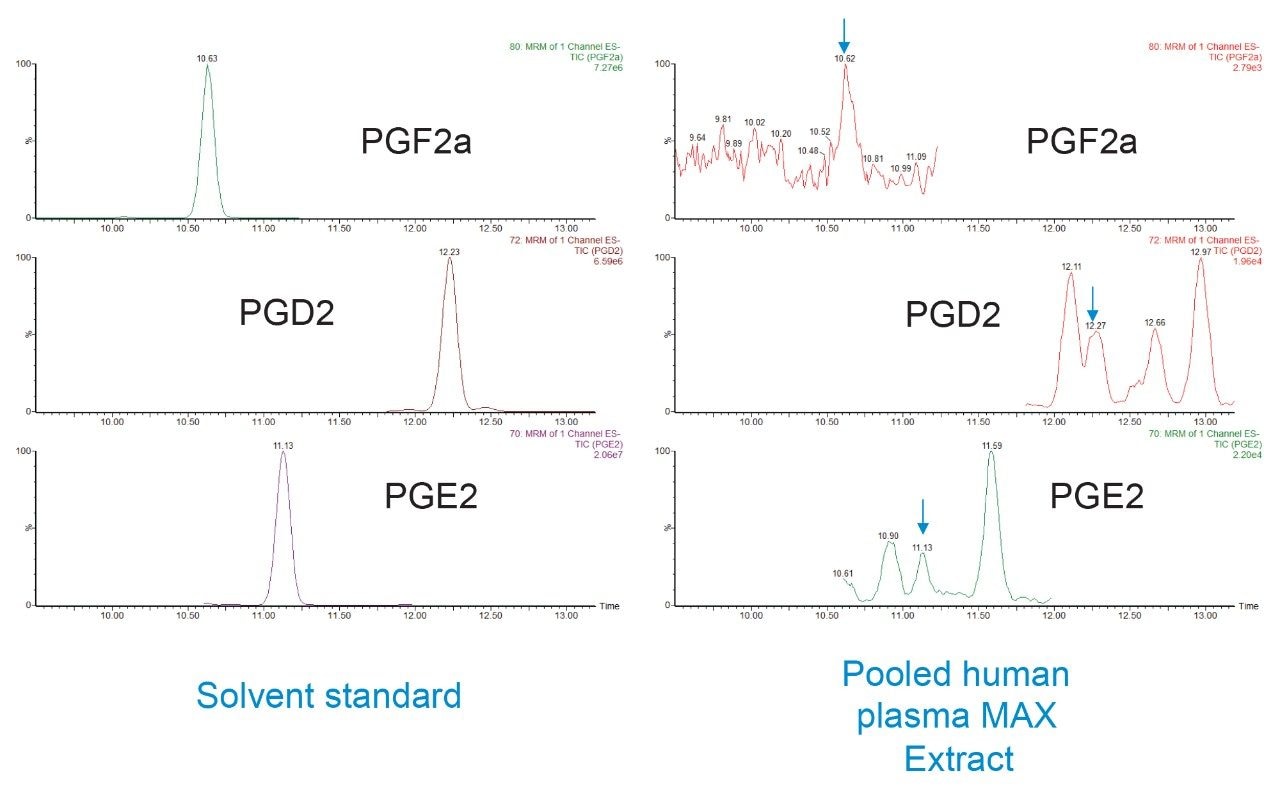
As a class of compounds, oxylipins have a particularly high number of structurally similar, and therefore isobaric, compounds. To achieve separation of these species, having the correct chromatographic conditions is critical. The reversed-phase UPLC conditions used here were extensively optimized, comparing various mobile phase/stationary phase combinations in order to obtain conditions that allowed separation of the multiple isobaric compounds. Figure 3 is a representative chromatogram from a pooled human plasma sample and shows the elution order of the most abundant oxylipins in the sample. Table 1 gives a comprehensive list of all the retention times and optimized MRM conditions for all 92 analytes. Figure 4 is an example of where the excellent specificity of the UPLC gradient is demonstrated. As is clear from the chromatograms shown, the ability to separate 2 structural isomers (i.e. PGE2 and PGD2) from a solvent standard is not sufficient as the matrix contains even more isobaric forms of these same compounds. The UPLC chromatograms shown for PGE2 and PGD2 in human plasma show just how many isobars there are for these analytes (the arrows indicate the analyte peaks).
Using a Xevo TQ-XS in negative ESI-mode, retention times and optimal MRM transitions were determined for all individual oxylipins (Table 1). To enhance the sensitivity of detection, these MRM transitions were monitored in defined retention time windows, maximizing dwell times by reducing overlapping transitions. Using this UPLC-MS/MS method, we rapidly profiled 92 oxylipins in plasma, serum, and cell culture samples.
A UPLC-MS/MS methodology has been developed for the research analysis of 92 oxylipins in human plasma/serum and cell culture samples. This method has been demonstrated to be suitable for the analysis of these oxylipins at physiologically relevant levels. The combination of specificities offered by mixed mode SPE extraction, UPLC separation, and tandem quadrupole mass spectrometry detection has produced a comprehensive analytical platform for the targeted analysis of oxylipins in biomedical research studies.
720007030, October 2020