
This is an Application Brief and does not contain a detailed Experimental section.
When equipped with p-QSM and ACQUITY BEH column technology, the ACQUITY APC System equips researchers with a 100% aggressive solvent separation solution that is compatible with organic solvents straight out-of-the-box. The p-QSM pump has the flexibility of running in isocratic mode or gradient mode.2 GPEC uses gradient elution for differentiation by solubility and chemistry. This Gradient elution technique is not to be confused with reverse phase separations or size based GPC separations, because the polymer is purposely precipitated onto the column with a “poor” solvent without interacting with the column and then dissolved off the column with a “good” solvent concentration in the gradient. GPEC requires a detector that can detect a polymer in a variable mobile phase and one that may not have a chromophore. ELSD works well under these conditions and with the GPEC technique.3-5 The purpose of this application brief is to differentiate various Stereochemical polylactic acid samples by solubility using gradient polymer elution chromatography (GPEC) and an ACQUITY Advanced Polymer Chromatography (APC) System with polymer quaternary solvent manager (p-QSM) and evaporative light scattering detection (ELSD).
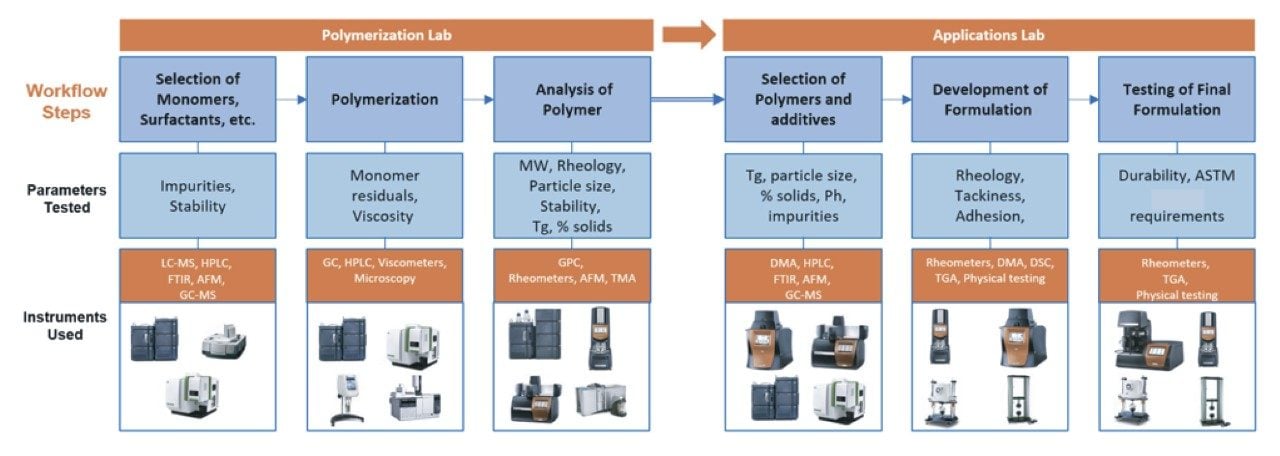
There are many varieties of polylactides or Polylactic acid (PLA) available from manufacturers and chemical suppliers. PLA variations can include molecular weight, stereochemistry, end group termination, and degree of crystallinity.1 To produce a PLA sample and control these variable characteristics, the raw materials, synthesis, and post synthesis treatments need to be controlled. Quick and simple analytical techniques are required to accurately measure the polymer during research, testing and final product formation; this process is captured in the PLA workflow in Figure 1. To enable researchers to confirm consistent polymer quality and performance, frequent analytical measurements are taken throughout the workflow.
In this application brief, we demonstrate how the highly solvent compatible capabilities of the ACQUITY APC with p-QSM and ELSD enables researchers with a quick and robust solution for applying the GPEC technique to differentiate PLA stereochemistry.
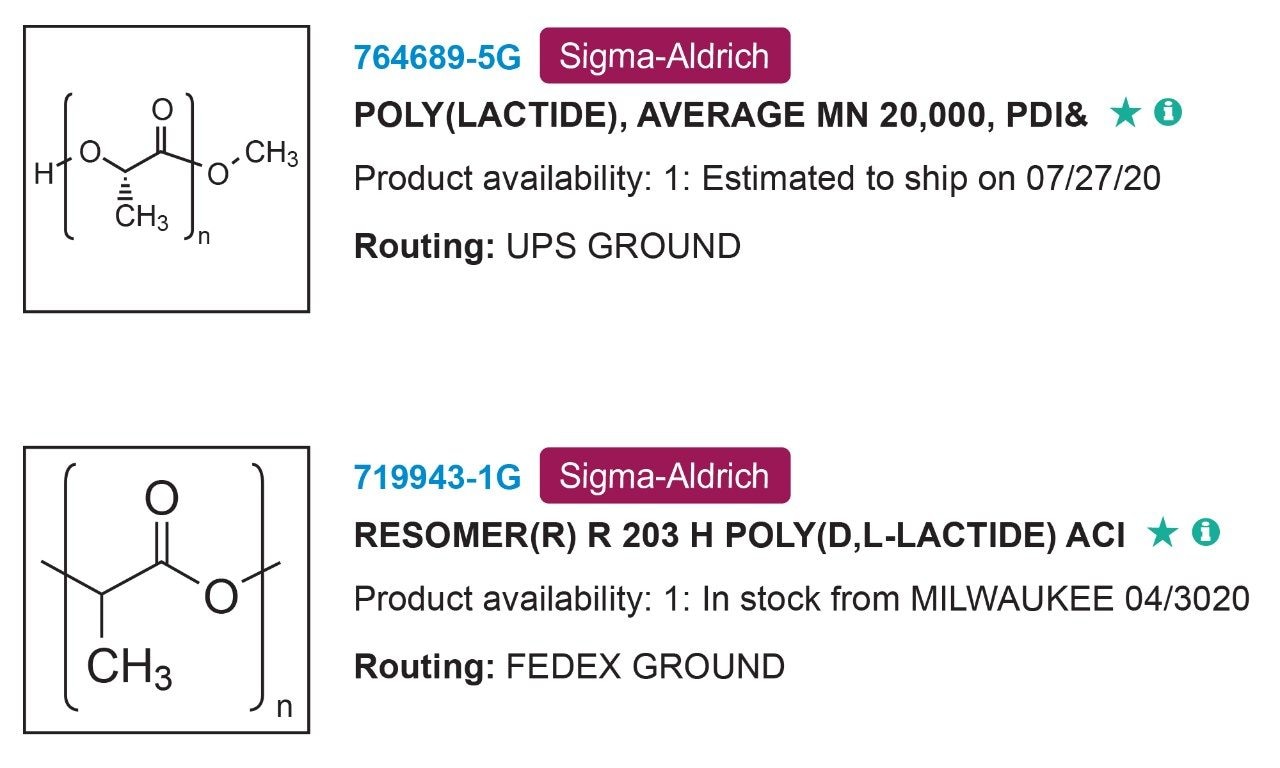
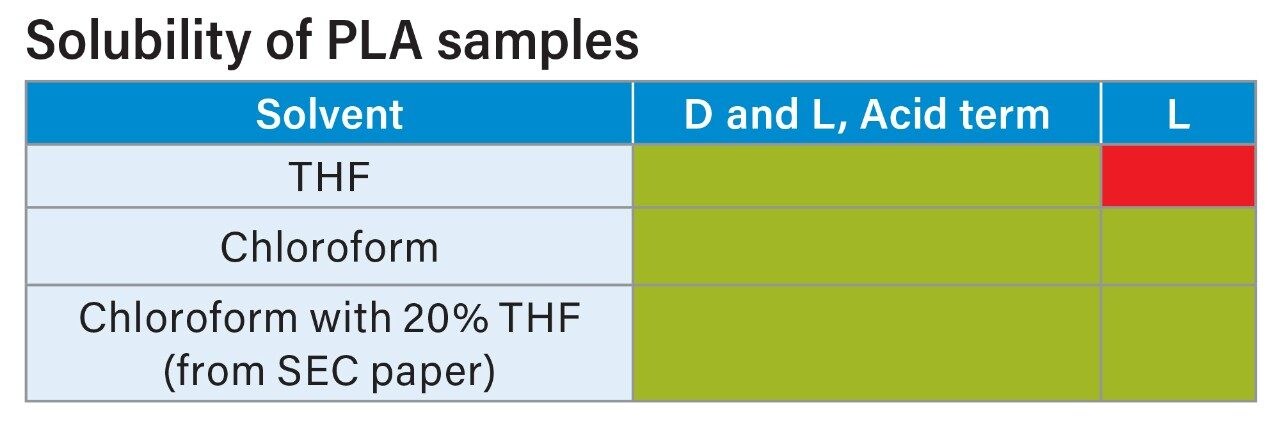
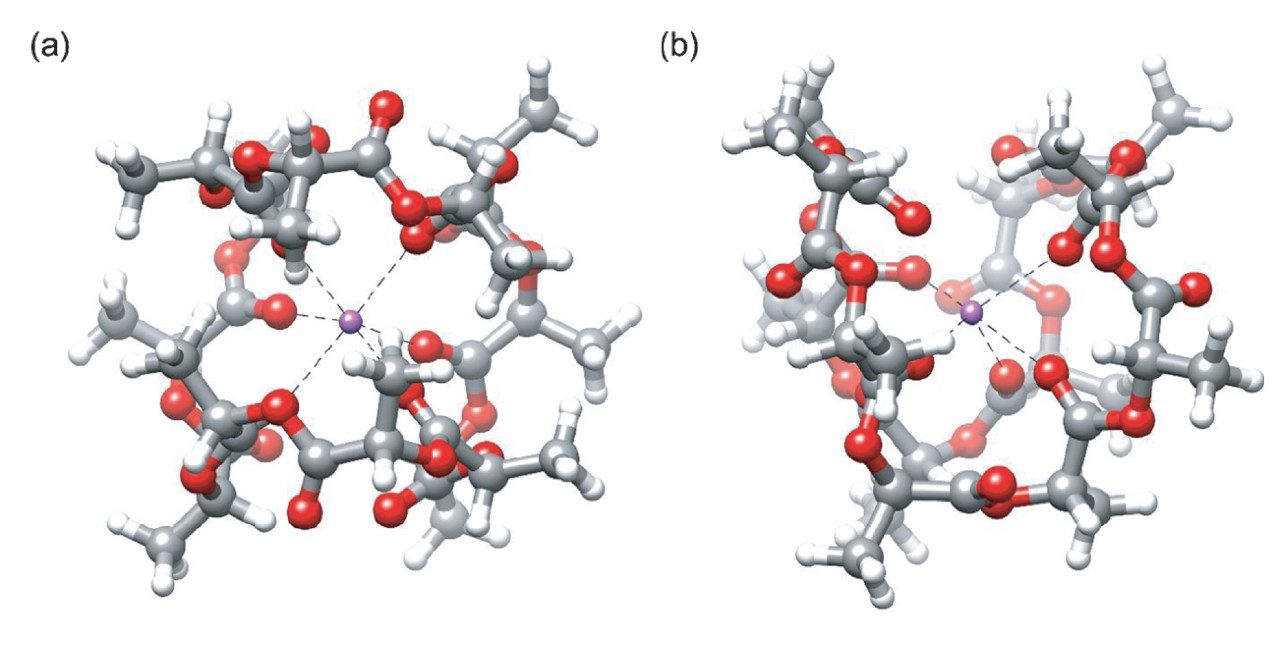
In this experiment, the PLA samples were purchased from Millipore Sigma. The samples were chosen to fall within a similar molecular weight range and with differing stereochemistry as highlighted in Figure 2. The first step in the process was determining solubility of the polymer samples. Using a PLA gel permeation chromatography (GPC) experiment as a starting point for dissolving the polymer samples, a solubility study is completed in Table 1.4 Polymer solubility can be related to molecular symmetry and the ability to form associations leading to crystallinity (Figure 3). The PLLA form has more symmetry than the PLDLA form and symmetry aids associations and impedes solubility.
The PLA samples are dissolved in chloroform at a concentration of 5 mg/mL as a stock solution, are further diluted to 1 mg/mL in a separate vial, and then filtered through a PTFE 0.2 um, 13 mm syringe filter (P/N WAT200556). The filtered sample is placed in a Waters 2 mL liquid chromatography vial with a pre-slit septa cap (P/N 186005666CV).
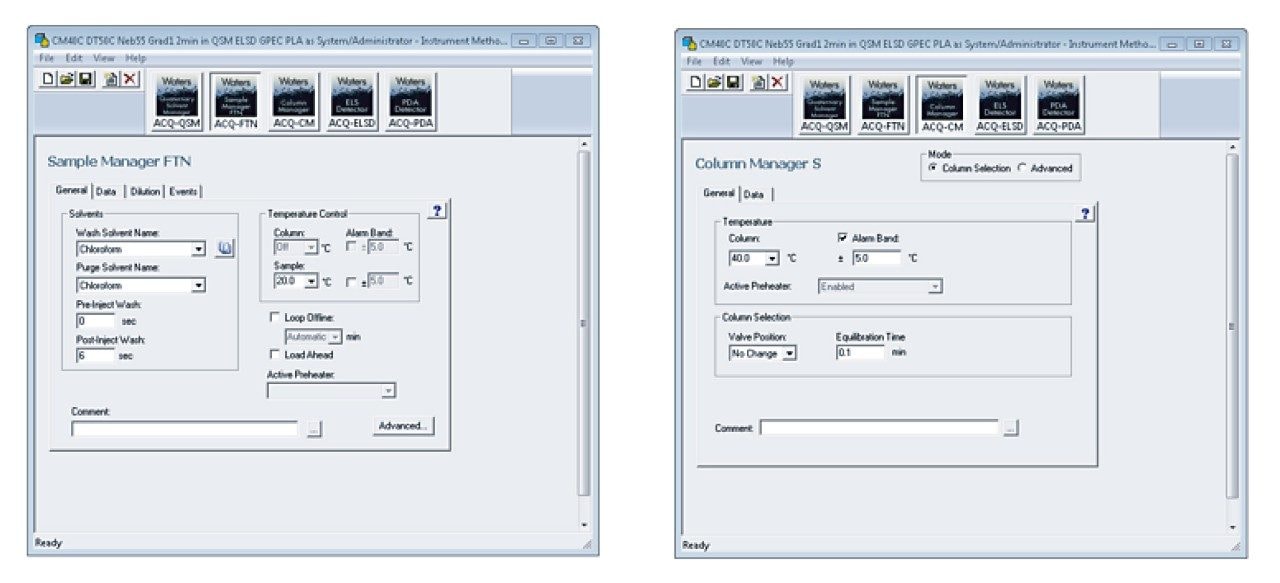
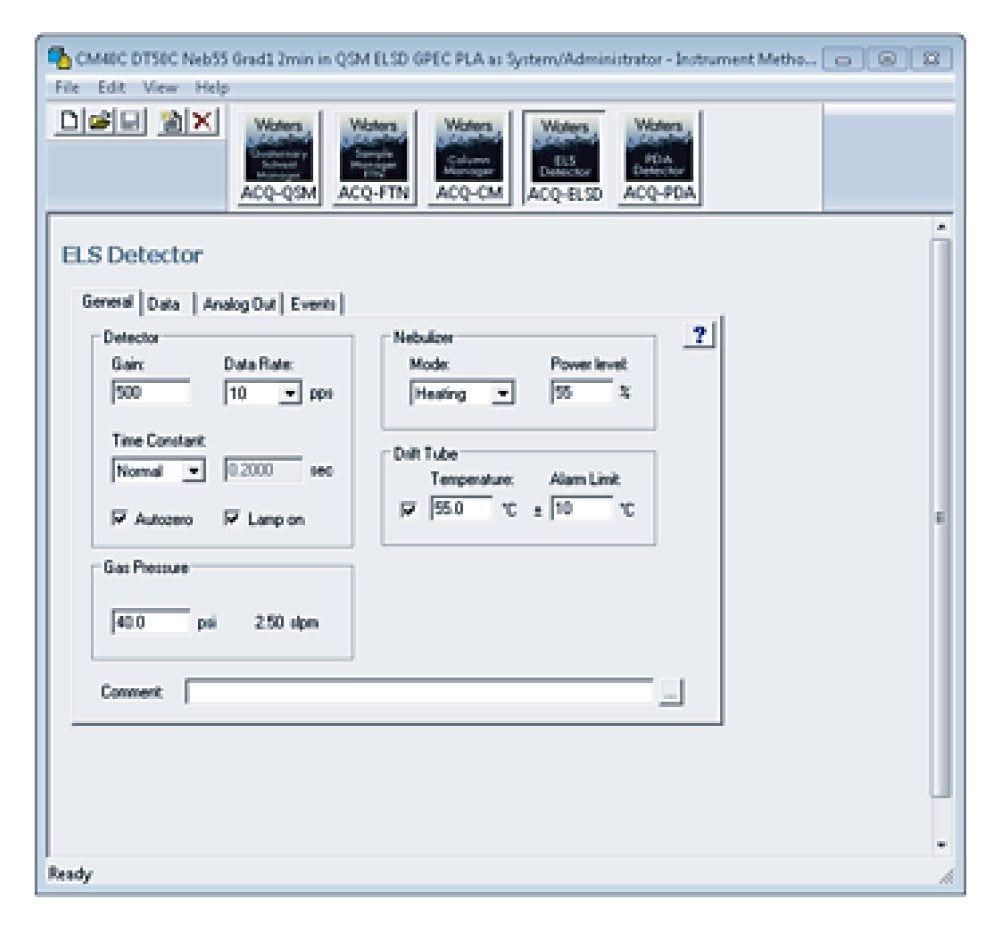
Once the samples are prepared, the column is installed (P/N 186006046), the mobile phases are placed on top of the instrument, and the instrument settings are entered in the Empower instrument control software. Experimental details are captured in Table 2 with the Empower Scientific Software screenshots of the ACQUITY APC System instrument settings highlighted in Figures 3 through 5.
|
System: |
APC with p-QSM (P/N 176015030) |
|
Column: |
XBridge BEH C8 XP Column, 130Å, 2.5 µm, 3 mm x 75 mm |
|
Mobile phase A/B: |
Chloroform |
|
Mobile phase C/D: |
Methanol |
|
Gradient: |
See screen shot |
|
Flow rate: |
0.7 µL/min |
|
Run time: |
10 min |
|
Injection volume: |
1 µL |
|
Sample conc.: |
1 mg/mL |
|
Detector: |
ELS |
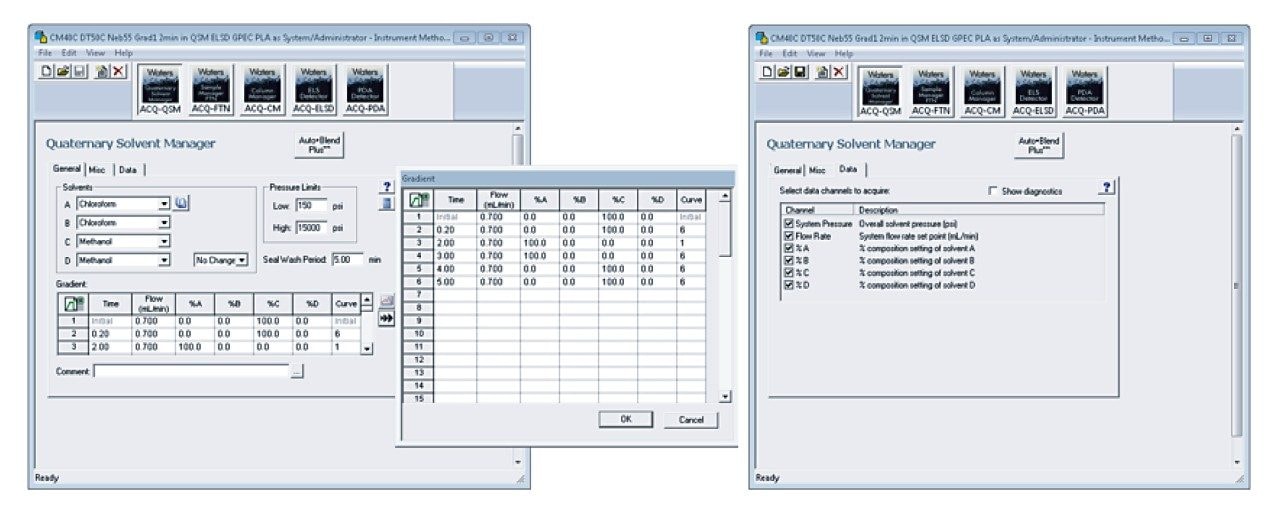
The resulting data from the experiment is put into review by selecting the %A gradient, PLA(L), and PLA(D,L). By selecting the overlay view and stacking the plots, the chromatograms are exhibited in Figure 7. The slope of the gradient is 2, because this is a very steep and quick addition of the “good” solvent chloroform. By adding the chloroform quickly, the polymer rapidly dissolves off the column and yields a narrow chromatographic peak. The PLA samples elute at different times due to their solubility, gradient solvent ratio, and stereochemistry.
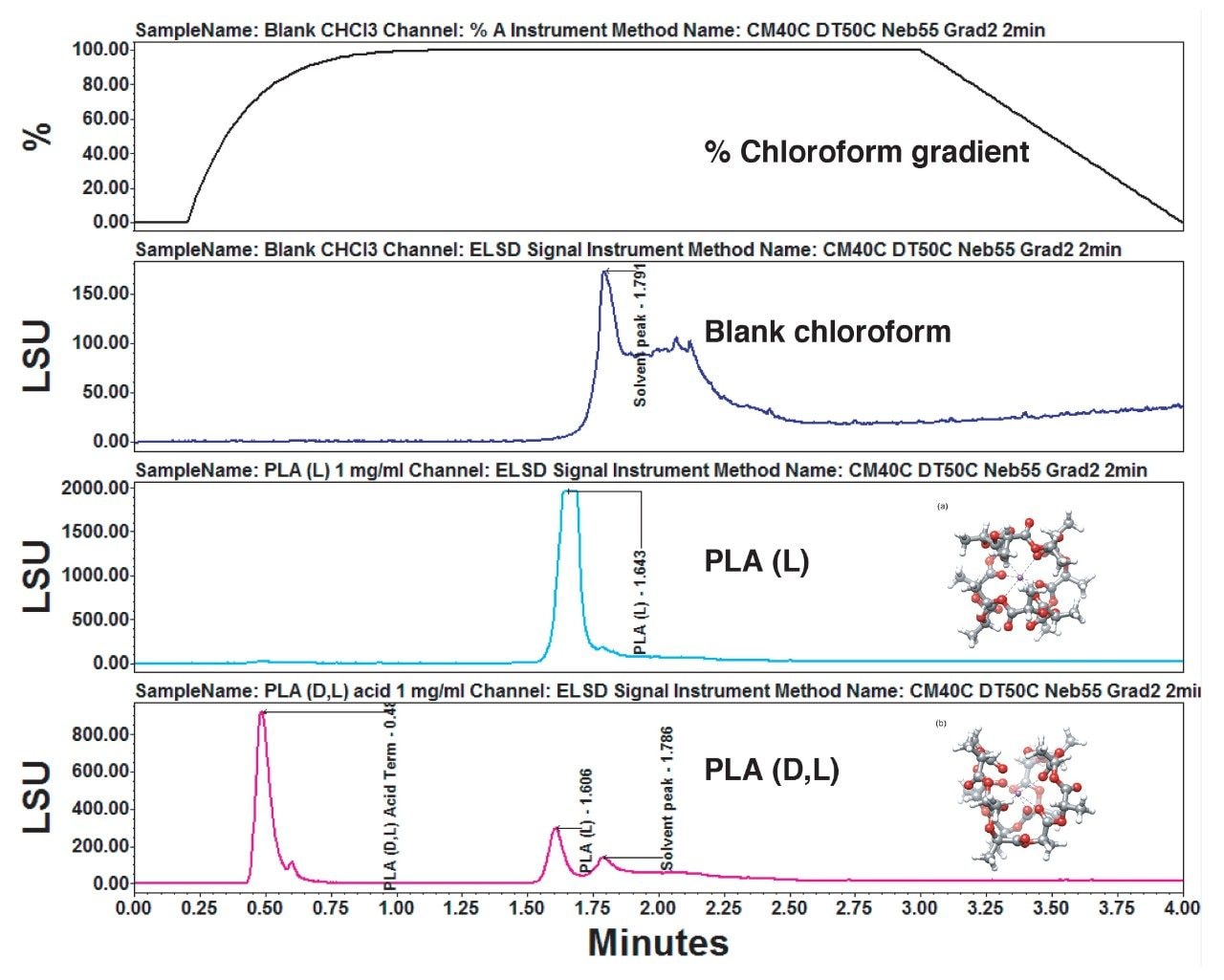
Using an ACQUITY APC System with p-QSM and ELSD, two PLA samples of different stereochemistry undergo chromatographic separation by solubility and elute at different retention times by using the GPEC technique. This GPEC example is the first in a series of PLA experiments that will be shared in a larger body of work. The larger document will address the structures and properties associated with various PLA stereochemistry samples.
720006942, June 2020