Determination of Aflatoxin M1 in Milk Using Immunoaffinity Chromatography Cleanup Coupled With UPLC and Fluorescence Detection
Abstract
Aflatoxins are carcinogenic mycotoxins that have adverse health effects on both humans and animals consuming contaminated food and feed, respectively. Aflatoxin M1 (AFM1) is a metabolite of aflatoxin B1 (AFB1), which is present in the milk of animals that ingest feed contaminated with AFB1. A method has been developed for the highly sensitive and selective determination of AFM1 in milk. Samples were prepared by centrifuging the milk and separating out and removing the fat layer. The skim portion was then applied to the VICAM Afla M1 Immunoaffinity Chromatography (IAC) Column, which contains specific antibodies that selectively bind to AFM1. Once AFM1 is bound to the antibody on the column, the column was washed to remove matrix components and AFM1 eluted from the column. The AFM1 concentration was determined using an ACQUITY UltraPerformance Liquid Chromatography (UPLC) System with fluorescence detection. The performance of the method was evaluated through replicate analysis of spiked test portions. The limit of detection for this procedure was 0.005 µg/kg (ppb). Overall recovery was shown to be satisfactory, greater than 80%, with relative standard deviations lower than 10%. The method was found to be specific as no interference peaks were observed for blank samples. The method has been demonstrated as suitable for monitoring compliance with regulatory limits set for AFM1 in milk globally.
Benefits
- High performance – the method meets AOAC and European Commission method performance requirements
- Robustness – the method is a modification of an established AOAC official method
- Speed – the method requires only 2 mL of milk, so IAC cleanup is quick and UPLC offers short run times
Introduction
Mycotoxins are toxic fungal metabolites that are hazardous to human health and cause economic losses due to disease or reduced production efficiency in livestock. The most toxic and carcinogenic group of mycotoxins commonly found in food and animal feed is the aflatoxins. Milk and dairy products can contain aflatoxin M1 (AFM1), a metabolite of aflatoxin B1 (AFB1), a potent human carcinogen. AFM1 occurs in the milk of dairy animals ingesting feed contaminated with AFB1, which is partly converted to this hydroxylated metabolite and then excreted in milk.1 The presence of AFM1 in milk and dairy products is reported as a food safety concern worldwide for several reasons.2 AFM1 is categorized as a group 2B human carcinogen by the International Agency on Research on Cancer (IARC) and the acute toxicities of AFM1 and AFB1 are similar. AFM1 is heat stable, and normal processing and storage are not effective in reducing its levels in milk and dairy products. Small amounts of this contaminant may impose health risks for consumers of large quantities of milk products, such as children, a particularly vulnerable subgroup in the population. In addition, the economic consequences of AFM1 in milk and dairy products can be severe to dairy producers. A direct economic impact occurs when products that do not meet the aflatoxin standards are rejected at national or international markets. In some cases, the amount of AFB1 in animal feed and AFM1 in milk samples are comparatively high and can pose health hazards for local consumers. In two recent surveys in Bangladesh and Pakistan, 71% and 48% of samples were found to be contaminated with AFM1, respectively.2,3
Many countries have established strict regulations for aflatoxins in food including in milk, although these vary in different countries. For example, countries such as China, India, Russia, and USA, have adopted the maximum recommended Codex limit of 0.5 μg/L (ppb) for AFM1 in fluid milk products, whereas the EU has a lower limit of 0.05 μg/kg (ppb) AFM1 in milk.4 Milk is not only consumed as liquid milk, but also utilized for the preparation of infant formulas, yogurt, cheese, milk-based confectioneries including chocolate, and pastry.
It is crucial that the AFM1 concentrations in milk and dairy products are monitored, even in countries where regulations on food and feed contaminants are not yet in place. Immunochemical strip tests based upon the selectivity of monoclonal antibodies (e.g. VICAM’s Afla M1-V) are used for on-site testing, but chromatographic techniques are typically preferred for use in the laboratory and are used in official reference methods, often after immunoaffinity chromatography (IAC) column cleanup. High-performance liquid chromatography connected to a fluorescence detector (HPLC-FLD) is widely used for the determination of aflatoxins in food products, principally due to its great sensitivity. Based on the natural fluorescence exhibited by AFM1, FLD is more sensitive and selective than other optical detectors and does not require the enhancement through post-column derivatization needed for determination of some of the other aflatoxins.
The objective of this study was to demonstrate the performance of a method using the VICAM Afla-M1 LC IAC Column, with an ACQUITY UPLC System, for the determination of AFM1 in milk using a modification of the AOAC Official Method 2000.08.5,6 This method has been adopted for use for official control in many countries (e.g. India Method No. FSSAI 07.014:2020).7
Experimental
An overview of the details of sample extraction and cleanup for aflatoxins is given in Figure 1. Test portions were prepared by warming liquid milk in a water bath and then gently stirring with magnetic stirrer to disperse the fat layer prior to centrifugation. The upper fat layer was discarded, and the defatted (skim) milk was filtered prior to further analysis. The original AOAC official method specified a 50 mL test portion of skim milk but newer methods, using more sensitive detectors and UPLC instrumentation, require a smaller amount of sample, reducing the time taken for the cleanup step. Portions of the skim milk were applied to the Afla-M1 LC IAC Column. The AFM1 binds to the antibody on the column. The column was then washed with a mixture of water and methanol to remove co-extractives from the column, prior to elution of the AFM1 with methanol. Subsequent determination of AFMI in the extract was by UPLC with FLD. More details on the use of the Afla-M1 LC IAC Column can be found here.
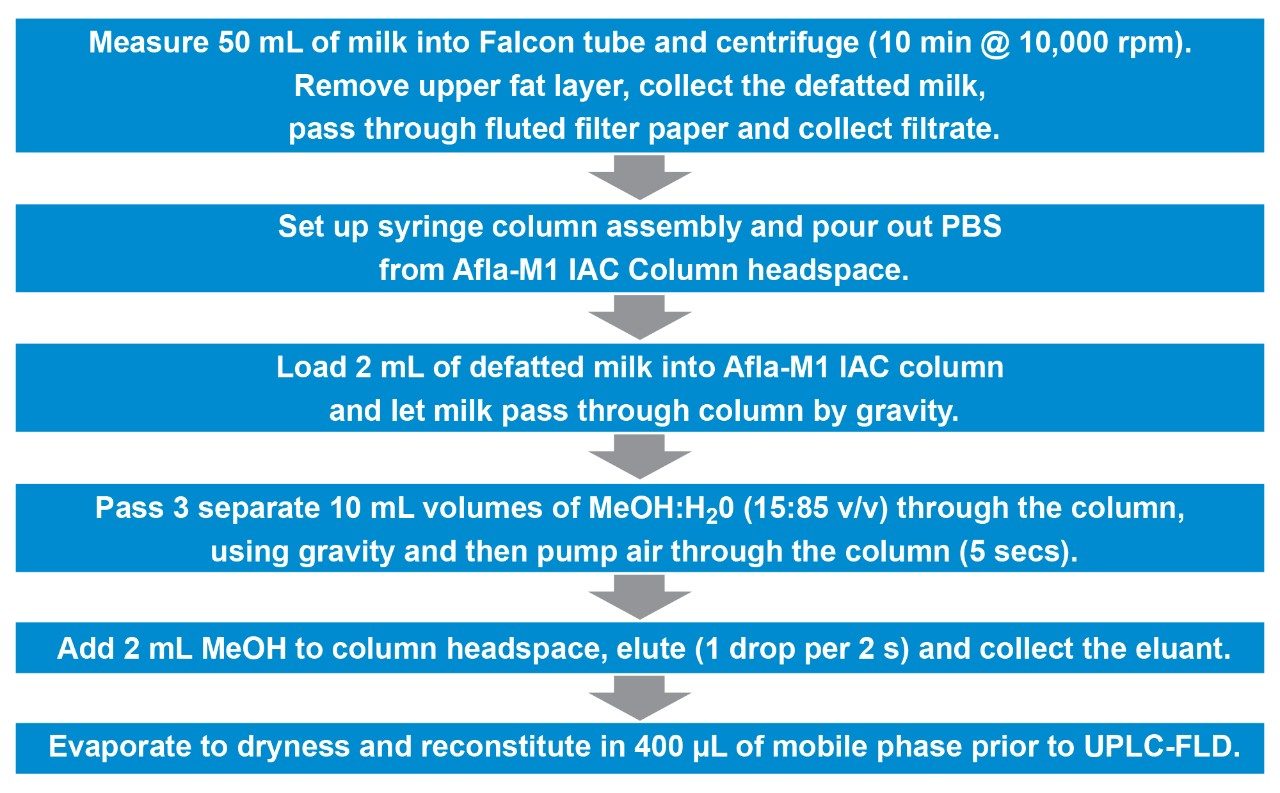
An AFM1 solution at 10 µg/mL in acetonitrile from Supelco (p/n CRM46319) was used to prepare the calibration standards and spiked milk samples. The calibration standards were prepared over the range 0.005 to 2.0 ng/mL by dilution with the mobile phase.
LC Conditions
|
LC system: |
ACQUITY UPLC H-Class PLUS with FTN Sample Manager |
|
Detection: |
ACQUITY Fluorescence Detector with large volume cell (p/n: 205000609); Excitation 360 nm: Emission 440 nm |
|
Vials: |
LCGC Certified Clear Glass Screw Neck Vial, 12 x 32 mm, 2 mL (p/n: 186000307C) |
|
Column: |
ACQUITY UPLC HSS T3 1.8 μm, 2.1 x 100 mm (p/n: 186009468) |
|
Column temp.: |
25 °C |
|
Sample temp.: |
25 °C |
|
Injection volume: |
10 µL |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
Water (68%, v/v) |
|
Mobile phase B: |
Acetonitrile (24%, v/v) |
|
Mobile phase C: |
Methanol (8%, v/v) |
|
Run time: |
3.5 min |
Data Management
|
Chromatography software: |
Empower 3 |
Method Validation
Validation was performed by replicate analysis of spiked milk samples, previous found to be blank. The following parameters were assessed: sensitivity, selectivity, linearity, trueness, and within-laboratory repeatability (RSDr). Trueness and repeatability were determined from the analysis of ten replicates prepared at three concentrations; the expected LOQ (0.005 µg/kg), the EU limit (0.05 µg/kg) and the Codex limit, adopted in many other countries including the USA (0.5 µg/kg).
Results and Discussion
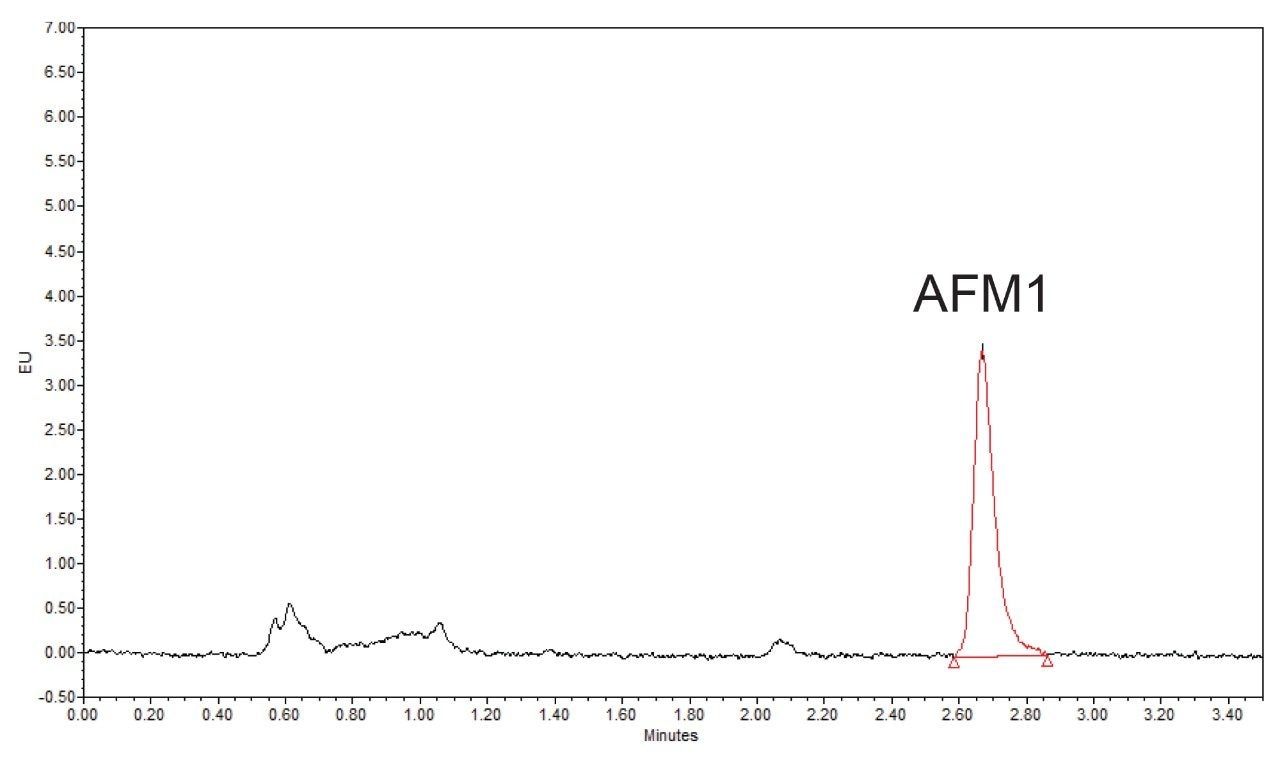
HPLC-FLD, using isocratic conditions, has been employed for the detection of AFM1 for many years. The combination of increased chromatographic efficiency from the sub 2 µm particles and UPLC system and use of a large volume flow cell within the Waters ACQUITY Fluorescence Detector provided very low limits of quantification. A typical UPLC chromatogram, using isocratic conditions is given in Figure 2. The chromatographic method provided excellent retention and peak shape for AFM1 within 3.5 minutes.
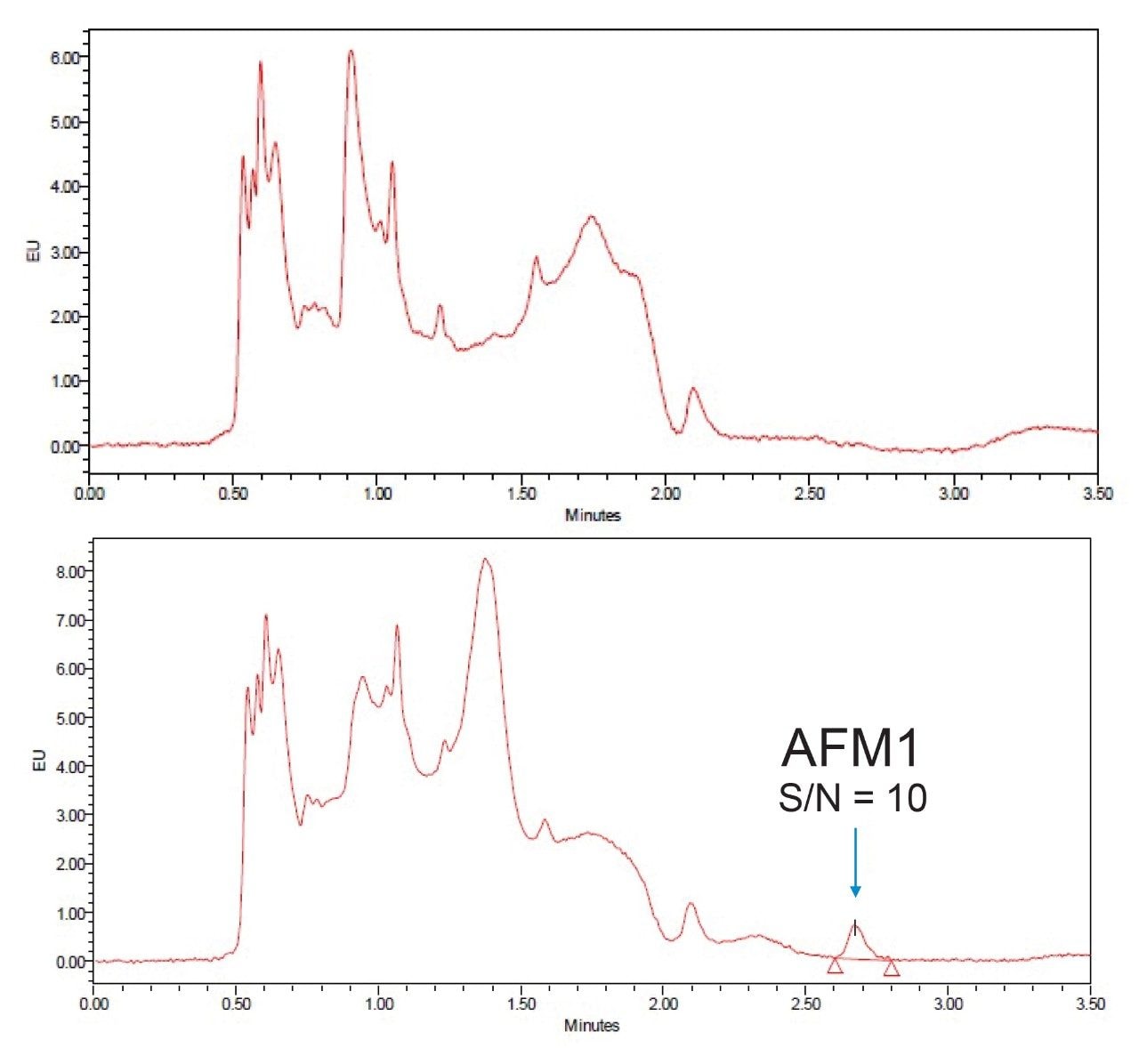
The sensitivity, as shown by the signal-to-noise ratio (S/N) for the peak in the chromatogram below (Figure 3), shows that the method is suitable for checking compliance with regulatory maximum limits worldwide. The limit of detection for this procedure is estimated at or below 0.005 µg/kg. No signals were detected in the extracts from the blank samples that could lead to detection of false reporting of non-compliant samples. For example, one can compare chromatograms from the analysis of milk in Figure 3.
A 4-point calibration curve, over the range 0.005 to 2.0 ng/mL, was prepared in mobile phase and was used to quantify the AFM1. A linear fit was applied and the value for the coefficient of determination (r2) of the curve was 0.9995 demonstrating reliable quantification for AFM1.
The trueness, expressed by percentage recovery, was within the range 80 to 114% and the repeatability (RSDr) was excellent, with values of 9.1, 9.3, and 2.4% RSD (see Table 1). These values were within the requirements defined by the European Commission8 and AOAC9.

Conclusion
The Afla M1 IAC Cleanup Column has been shown to remove potential interference from the milk in a timely manner, with good analyte recovery and precision. The ACQUITY UPLC H-Class PLUS option provided an opportunity to shorten the analytical run time and the use of a large volume flow cell in the fluorescence detector improved sensitivity. This method has the required sensitivity, selectivity, and overall performance to be used to check compliance with regulatory limits for AFM1 in milk worldwide.
References
- Koser P et al. The Genetics of Aflatoxin B1 Metabolism. J Biol. Chem. 1988 263: 12584–12595.
- Sumon A et al. The Presence of Aflatoxin M1 in Milk and Milk Products in Bangladesh. Toxins 2021, 13:440.
- Waqas N et al. Assessment of Aflatoxin B1 in Animal Feed and Aflatoxin M1 in Raw Milk Samples of Different Species of Milking Animals From Punjab, Pakistan. J Food Safety 2021 41(3):e12893.
- Turna N and Wu F. Aflatoxin M1 in Milk: A Global Occurrence, Intake, and Exposure Assessment. Trends in Food Science & Technology 2021 110:183–192.
- Dragacci S et al. Immunoaffinity Column Cleanup With Liquid Chromatography for Determination of AflatoxinM1 in Liquid Milk: Collaborative Study. J AOAC Int 2001 84(2):437–443.
- AOAC Official Method 2000.08-2004. Aflatoxin M1 in Liquid Milk. Immunoaffinity Column by Liquid Chromatography; AOAC International: Rockville, MD, USA, 2004, pp. 1–3.
- FSSAI. Manual of Methods of Analysis of Foods–Mycotoxins. 2020. https://fssai.gov.in/upload/advisories/2020/12/5fca3db8df192Order_Revised_Manual_Mycotoxins_04_12_2020.pdf.
- European Union. Commission Regulation (EC) No 401/2006 of 23 February 2006 Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs. Off. J. Eur. Union 2006, L 70:12–34.
- AOAC. Official Methods of Analysis. Appendix F Guidelines for Standard Method Performance Requirements, 2016.
720007431, November 2021