
A reproducible high sensitivity UPLC-MS/MS bioanalytical methodology is demonstrated using the Xevo TQ-XS Tandem Quadrupole Mass Spectrometer. A simple protein precipitation sample preparation was used for the detection of Midazolam and Imipramine down to 250 ag on column (0.2 pg/mL). This limit of detection was also shown to be reproducible across three separate UPLC-MS/MS systems.
Bioanalysis forms a critical part of the drug discovery and development process, providing information on pharmacokinetics and dose response. Lower doses of more potent drug candidates, along with reduced sample sizes from more ethically driven studies (reduce, replace, refine) mean that LC-MS method sensitivity is increasingly important. Concurrent with this requirement for increased sensitivity is the need to seamlessly transfer assays between laboratories, either within an organization or to a contract analysis organization. Thus, not only is MS sensitivity important, so too is instrument to instrument reproducibility.
In this application note we demonstrate the reproducible, robust sensitivity achievable using the Xevo TQ-XS Tandem Quadrupole Mass Spectrometer as part of a UPLC-MS/MS system for bioanalytical studies. Sensitivity limits as low as 250 ag (0.25 femtograms) of Midazolam and Imipramine were detected on-column from a matrix sample (human plasma) using a rudimentary, generic sample preparation technique (protein precipitation). This level of sensitivity was demonstrated to be reproducibly achievable across three separate UPLC-MS/MS systems. Intra and inter instrument precision were also calculated for both compounds on all systems in order to calculate limits of quantification.
Control human plasma was used to create calibration curves and QC samples containing both midazolam and imipramine at various concentrations. A combined 10 ng/mL stock solution was prepared in 50:50 methanol:water by diluting methanolic 1 mg/mL solutions of each of the compounds, these solutions were used as spiking solutions to create the calibration lines and QCs and were purchased from Sigma Aldrich (UK). Three separate UPLC-MS/MS systems were used to analyze freshly prepared calibration lines (0.2–100 pg/mL) and 12 QC samples (4 x 0.2 pg/mL, 4 x 0.4 pg/mL and 4 x 1.0 pg/mL). These samples were prepared using protein precipitation as follows. 100 µL of sample was precipitated using 300 µL of acetonitrile. Following centrifugation at 25,000 g the supernatant was diluted 1:1 using deionized water. 10 µL of this was then injected onto the UPLC-MS/MS system.
|
LC Conditions |
|
|---|---|
|
LC system: |
ACQUITY UPLC I-Class |
|
Detection: |
Xevo TQ-XS |
|
Vials: |
1 mL 96-well plate |
|
Column(s): |
ACQUITY, 2.1 x 50 mm, C18, 1.7 µm |
|
Column temp.: |
55 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
10 µL |
|
Flow rate: |
0.45 mL/min |
|
Mobile phase A: |
0.1% Formic acid in water with 10 mM Ammonium formate |
|
Mobile phase B: |
0.1% Formic acid in acetonitrile |
|
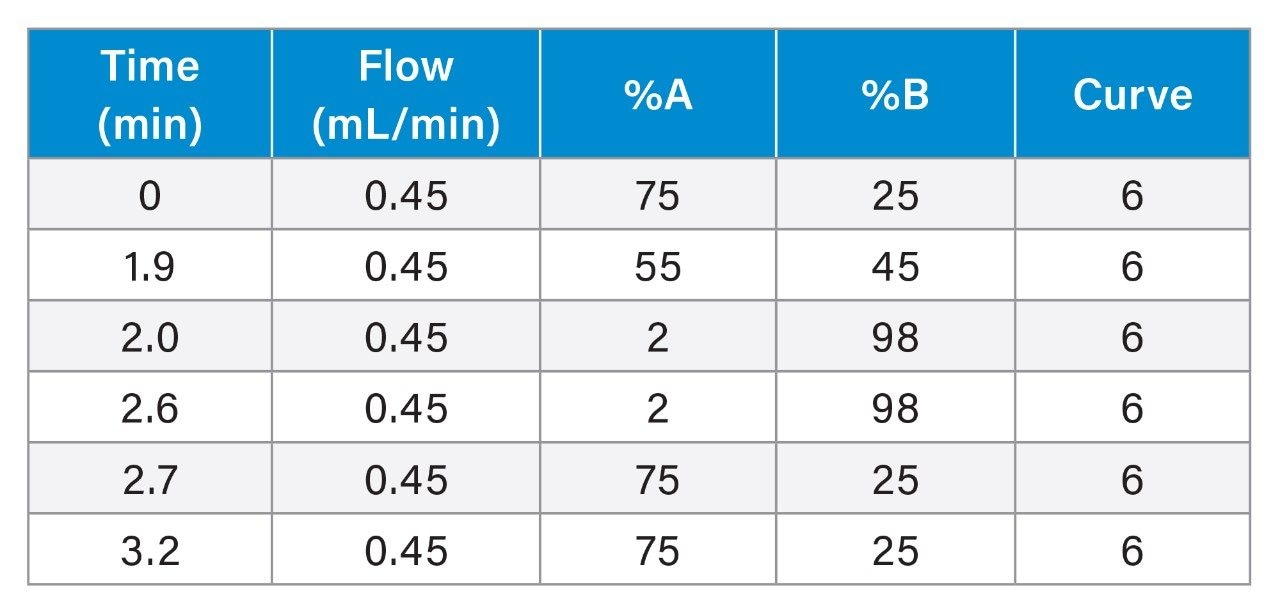
Gradient: |
Linear gradient from 25–45% mobile phase B over 1.9 min |
|
MS system: |
Xevo TQ-XS |
|
Ionization mode: |
ESI+ |
|
Acquisition range: |
MRM transitions: Midazolam - 326.1 > 291.0 (CV=60, CE=26) Imipramine - 281.1 > 86.0 (CV=30, CE=16) |
|
Capillary voltage: |
0.5 kV |
|
Collision energy: |
See above |
|
Cone voltage: |
See above |
|
Chromatography software: |
MassLynx v4.2 |
|
MS software: |
MassLynx v4.2 |
|
Informatics: |
TargetLynx XS v4.2 |
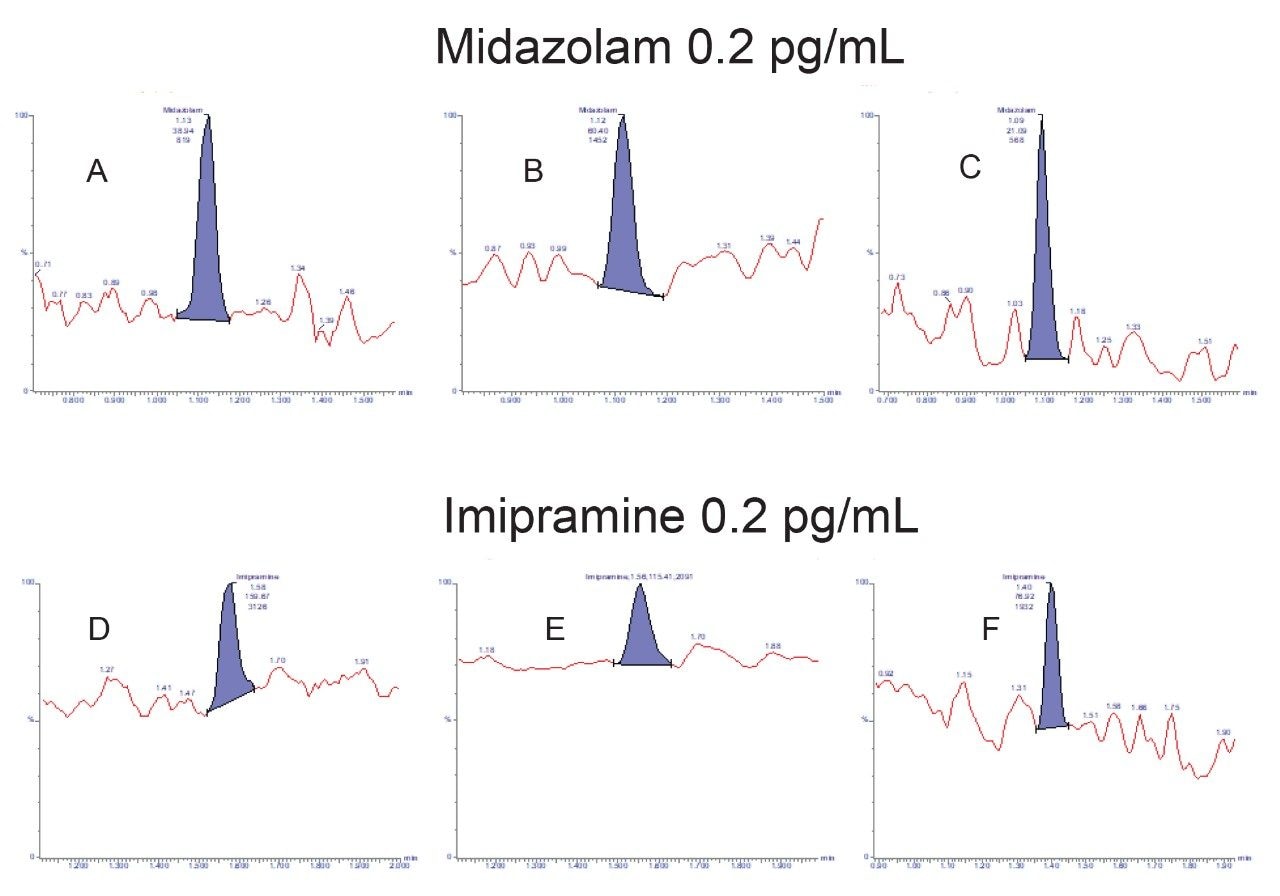
To evaluate the usable sensitivity and reproducibly of the methodology, calibration curves and QC samples were successfully analyzed using 3 separate UPLC-MS/MS systems. The three analyses were performed on consecutive days, the batch size and injection order was the same in all cases and consisted of the calibration line (0.2, 0.4, 1, 5, 10, 50, and 100 pg/mL) with blanks before and after, followed by the QC samples in order of increasing concentration, with blanks before and after. The three systems utilized an ACQUITY UPLC I-Class coupled to a Xevo TQ-XS Tandem Quadrupole Mass Spectrometer. The two target analytes were well retained on the generic chromatography system and were baseline resolved. The retention times for midazolam and imipramine were 1.1 and 1.6 min respectively. Calibration lines for both analytes on all three systems were linear over the full range of 0.2–100 pg/mL for both midazolam and imipramine, with r2 values of above 0.99 using 1/x weighting. The limit of detection for both analytes using all three LC-MS systems were determined to be 0.2 pg/mL for both analytes, with the lowest level calibrator and all four QC samples at that level being detectable in all cases (see Figure 1). Precision (%CV) for both analytes on each system at 0.2, 0.4, and 1.0 pg/mL was calculated to determine a limit of quantification for both analytes. The mean calculated concentration for all QC levels were accurate to within 15% of the nominal value for all analyses. Lower limits of quantification (LLOQ) were determined to be 0.4 pg/mL and 1.0 pg/mL for imipramine and midazolam respectively with overall, inter-system precision in both cases <8%. Figure 2 shows for imipramine how the %CV for each of the three systems varied with concentration.
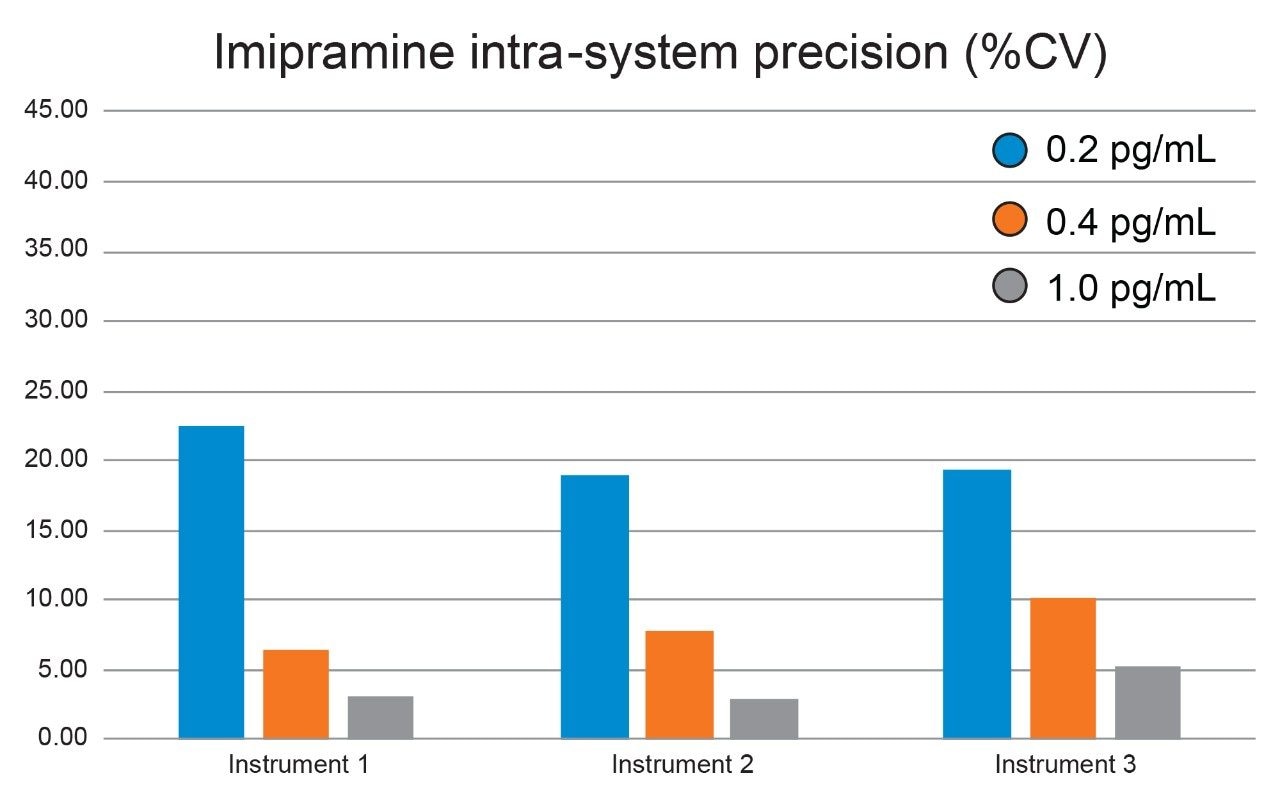
A high sensitivity UPLC-MS/MS bioanalytical methodology for the quantification of midazolam and imipramine in human plasma has been demonstrated using multiple Xevo TQ-XS Mass Spectrometers in combination with the ACQUITY UPLC I-Class. This method has been shown to have very high levels of sensitivity (250 ag on column), and this has been shown to be reproducible across systems. The limits of quantification and linearity have also been calculated and have also been shown to be reproducible across systems.
720007208, March 2021