This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates aspects of the GlycoWorks workflow including its flexibility to accommodate different SPE device formats and the availability of a human IgG control standard to use during method confirmation and troubleshooting.
GlycoWorks facilitates the preparation of N-linked glycans for analysis by providing the analyst with options in SPE device format (low/high-throughput) as well as an IgG control standard for method confirmation and troubleshooting.
Glycosylation of proteins is a highly significant post-translational modification that can modulate both protein structure and function. The glycosylation of biotherapeutics is understandably a structural feature that must be thoroughly characterized and monitored, particularly since changes in a glycan profile can correspond to changes in efficacy and/or immunogenicity.
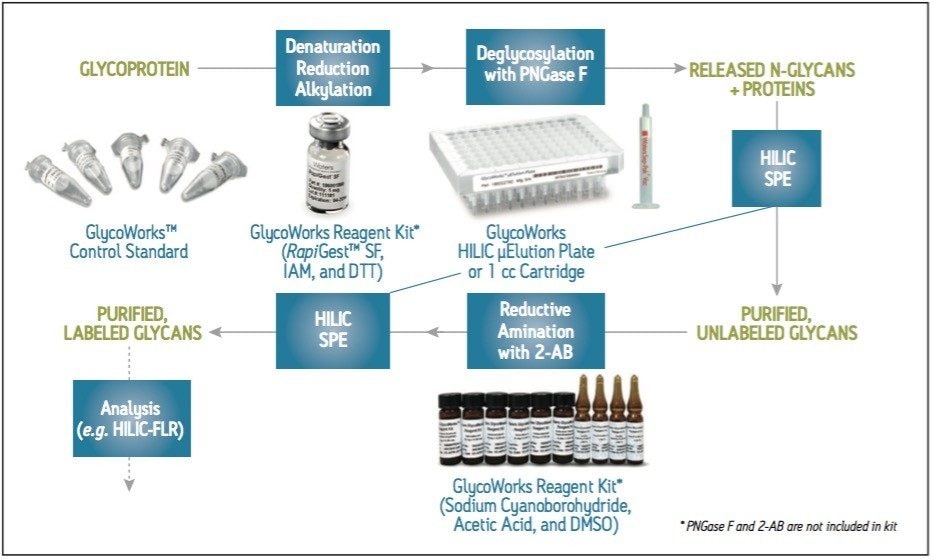
A commonly employed method for evaluating N-glycans from glycoproteins involves the release of glycans by PNGase F, their labeling with fluorescently active 2-aminobenzamide (2-AB), subsequent separation via hydrophilic interaction chromatography (HILIC), and detection by fluorescence (FLR) (Figure 1). Sample preparation can be complicated during this workflow. To make this process more straightforward, Waters introduced GlycoWorks, bringing together many of the consumables that are needed to prepare N-glycans for analysis. Most notably, GlycoWorks provides HILIC SPE devices for the pre- and post-labeling cleanup steps that are important for ensuring method robustness. The following work demonstrates two salient aspects of the GlycoWorks workflow: that it can accommodate the use of different SPE device formats (single-use cartridges and high-throughput plates), and that the included human IgG control standard can be used for method confirmation and troubleshooting.
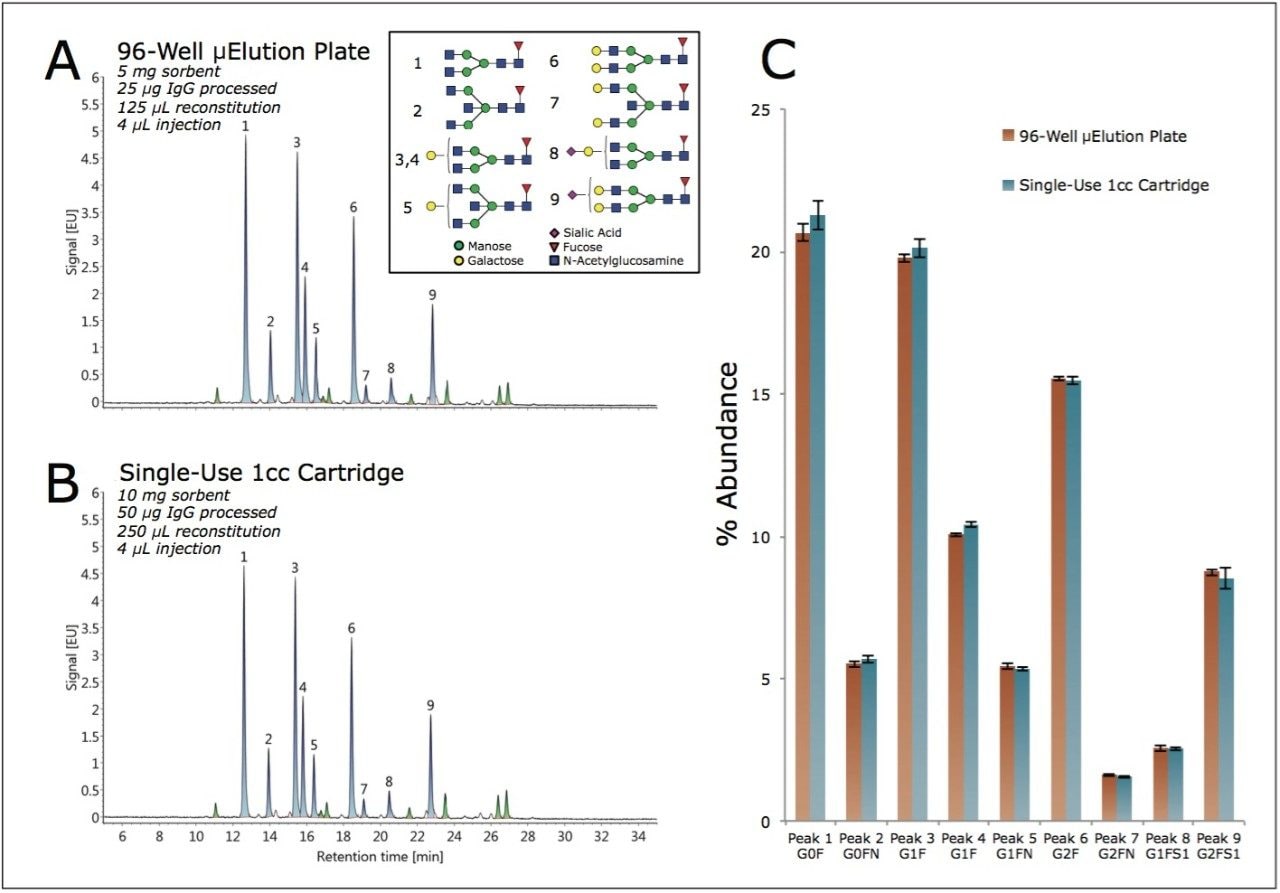
2-AB labeled N-glycans were prepared from the GlycoWorks Control Standard (p/n 186007033), a human IgG, using two different SPE device formats with protocols provided in their respective care and use manuals. Figure 2A shows a HILIC-FLR chromatogram obtained for the glycans of the Control Standard using the GlycoWorks HILIC μElution 96-well Plate (p/n 186002780), an ACQUITY UPLC GST Amide (BEH Glycan) Column, and UNIFI for instrument control and data interpretation. Assignments for nine of the most abundant species in this glycan profile were made by means of glucose unit (GU) values, and by comparisons to the Glycan Performance Test Standard (p/n 186006349) which, like the Control Standard, is based on human IgG. A chromatogram obtained when using GlycoWorks HILIC 1-cc Cartridges (single-use) (p/n 186007080) is shown in Figure 2B. Visual inspection of the two chromatograms indicated that highly similar results are obtained regardless of the chosen SPE device format. To confirm this, assigned peaks were integrated and peak areas were used to determine the relative abundances of the nine aforementioned glycan species (Figure 2C). This analysis revealed no significant differences in the relative levels of these glycans between samples prepared using the two different formats.
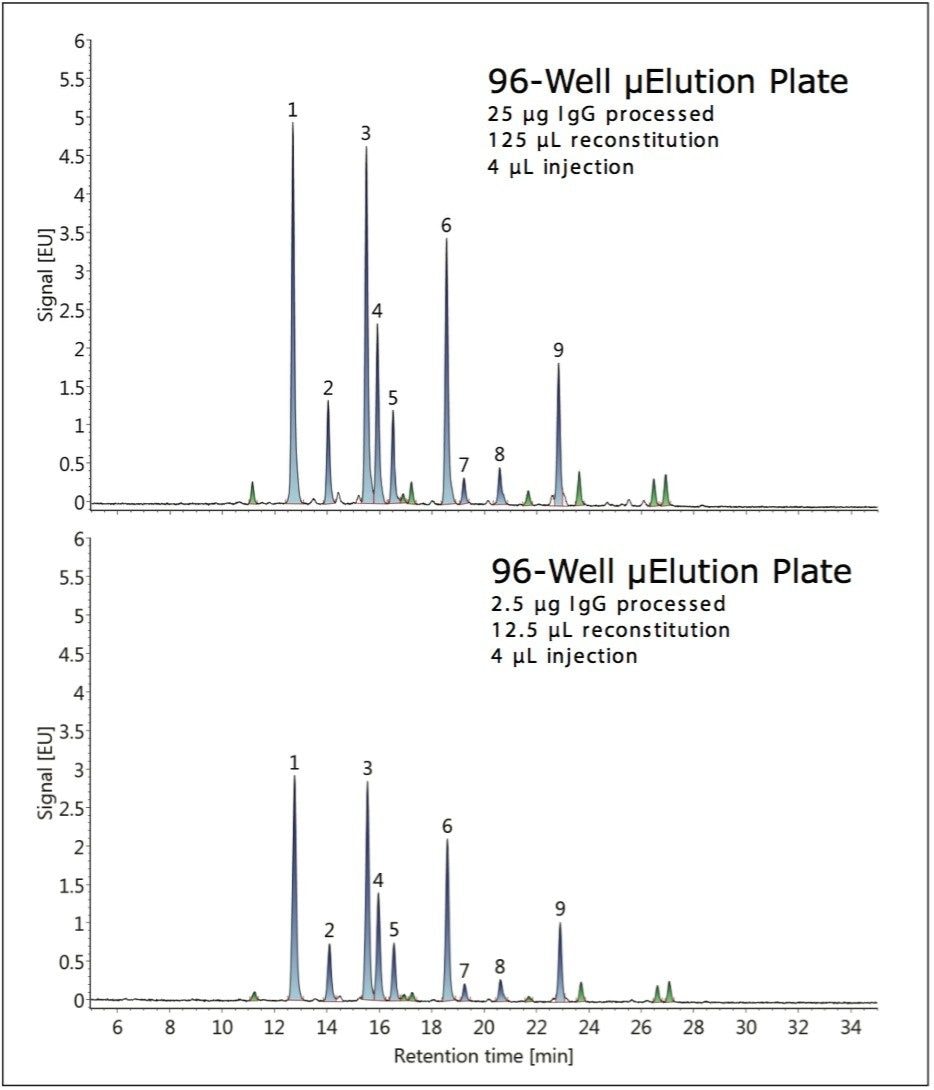
During this study, the GlycoWorks Control Standard was also employed to evaluate the effect of sample quantity on the glycan profile. HILIC-FLR chromatograms obtained when using the HILIC μElution Plate to prepare glycans from 25 μg and 2.5 μg of IgG are shown in Figure 3. Comparable results were obtained. However, the total recovery for the 2.5-μg sample was lower on average, likely due to low-level non-specific surface losses. The relative abundances were determined and found to be highly similar, differing by ≤11% despite the fact that the quantity of sample processed had changed ten-fold. The largest deviation corresponded to peak nine (G2FS1), which was observed to be present at a level of 8.5% versus 7.6% when processing 25 μg and 2.5 μg of IgG, respectively.
The GlycoWorks workflow accommodates the use of two different SPE device formats: one suited for high-throughput needs (96-well μElution plate) and another for low-throughput applications (1-cc cartridge). Most importantly, the results demonstrate that highly similar glycan profiles are measured regardless of the GlycoWorks SPE format chosen. The results were based on applying the GlycoWorks workflow to analyze the N-linked glycans of the Glycoworks Control Standard. This standard is included in the GlycoWorks Kits and may be used to confirm that the analysis is yielding appropriate results, or potentially aid in troubleshooting the procedure.
720004716, May 2013