In this application note, we illustrate and provide guidelines for fast, high resolution, and efficient analysis of therapeutic phosphorothioate oligonucleotides in a single analysis method.
Fast, high-resolution, efficient analysis of therapeutic phosphorothioate oligonucleotides in a single analysis method.
Antisense phosphorothioate oligonucleotide therapies are a promising treatment for a number of diseases, including cancer, diabetes, high cholesterol, and AIDS. The inherently unique characteristics of phosphorothioate oligonucleotides, combined with the multiple-step manufacturing process, make analysis of these oligonucleotides challenging. Post-purification analysis is a difficult and time-consuming process, typically requiring multiple orthogonal methods (CGE and SAX HPLC), adding significant costs and burden to a regulated QC laboratory.
The ACQUITY UltraPerformance LC (UPLC) System with Oligonucleotide Separation Technology (OST) Columns, packed with 1.7 µm sorbent, offer superior analytical performance for phosphorothioate oligonucleotide separations compared to HPLC and fast LC separations. As a result, method development and analysis are accomplished in dramatically shorter time, saving valuable time and analytical resources.
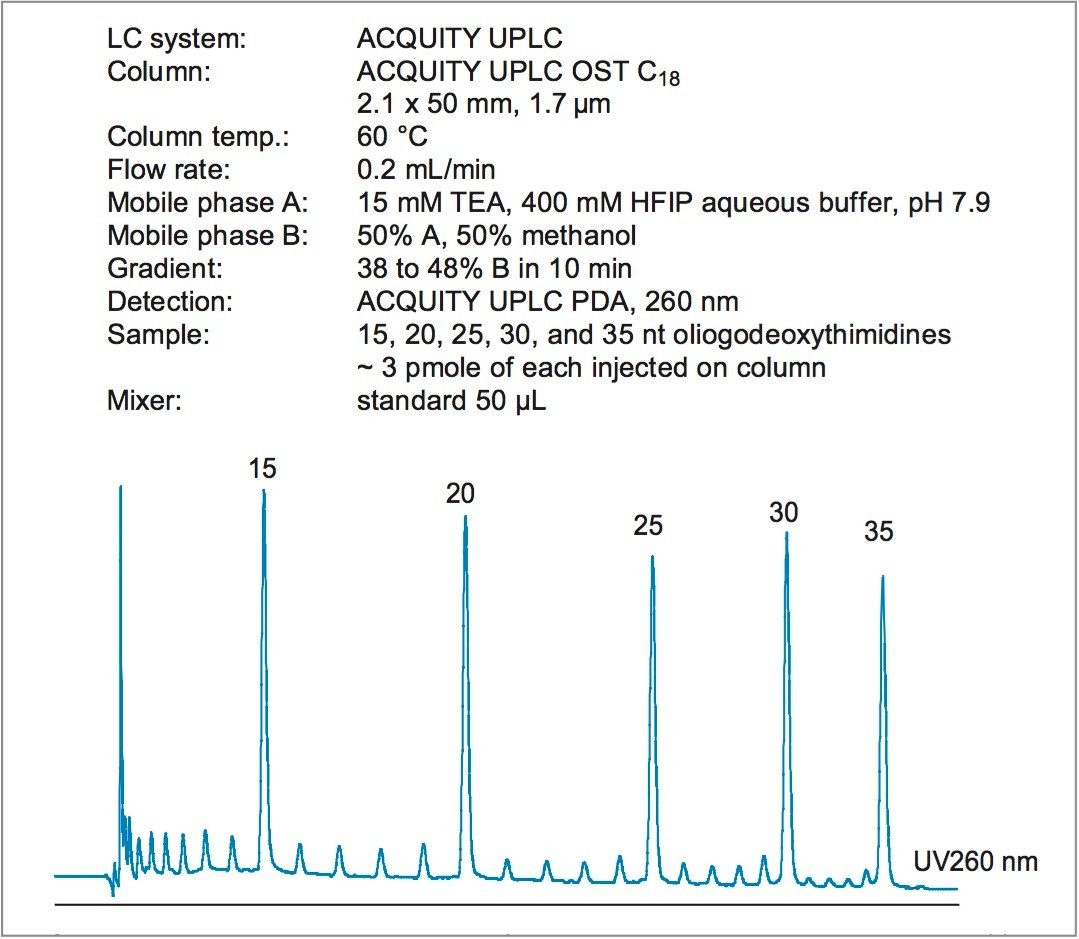
The ACQUITY UPLC System and OST Columns are used for fast and efficient separation of oligonucleotides using ion-pairing reversed phase liquid chromatography (IP-RP LC) mode. Figure 1 illustrates the oligonucleotide separation on the mix of 15, 20, 25, 30, and 35 nt oligodeoxythymidines. The minor peaks are by-products of failed synthesis. Baseline n-1 resolution of all species is achieved in less than ten minutes.
Phosphorothioate oligonucleotides are more difficult to analyze than phosphorodiester ones. When replacing an oxygen atom in the oligo backbone for sulfur, multiple diastereomers are created. Partial separation of isomers broadens the peaks in both capillary electrophoresis (CE) and liquid chromatography (LC), and complicates the analysis.
While the traditional triethylammonium acetate (TEAA) ion-pairing system is useful for phosphorodiester oligonucleotides, it fails when applied for separation of phosphorothioate oligonucleotides. Recently, Fountain and Gilar described a novel ion-pairing buffer suitable for efficient analysis of therapeutic phosphorothioate oligonucleotides.1,2 The buffer is comprised of triethylamine (TEA, an ion-pairing agent) and aqueous hexafluoroisopropanol (HFIP, a volatile weak acid used as buffering component to bring the pH to ~8). In addition, this ion-pairing system is compatible with both UV and electrospray MS detection.
The method development for oligonucleotide separation includes an optimization of gradient slope and initial mobile phase elution strength. The method development for analysis of modified oligonucleotides should reflect the fact that these are often more retained in IP-RP LC. An adjustment of initial mobile phase strength may be necessary, especially for 2'O-methylated oligos.
Figure 2 shows the separation of 25 nt phosphorothioate oligonucleotide that was partially hydrolyzed with snake venom phosphodiesterase (3'-exonuclease). The main 25 nt peak was clearly resolved from the N-x 3' truncated species. The identity of the peaks was confirmed by their mass (data are not shown).
The gradient slope used for phosphorothioate separation was 0.2% MeOH per minute. In order to maintain a smooth gradient profile when generating the gradient from 100% aqueous and 100% organic mobile phases, the larger mixer (425 µL) is recom-mended. Figure 2 illustrates that the analysis time can be reduced without sacrificing a resolution. This is achieved by appropriately adjusting the initial gradient strength while keeping the gradient slope constant.
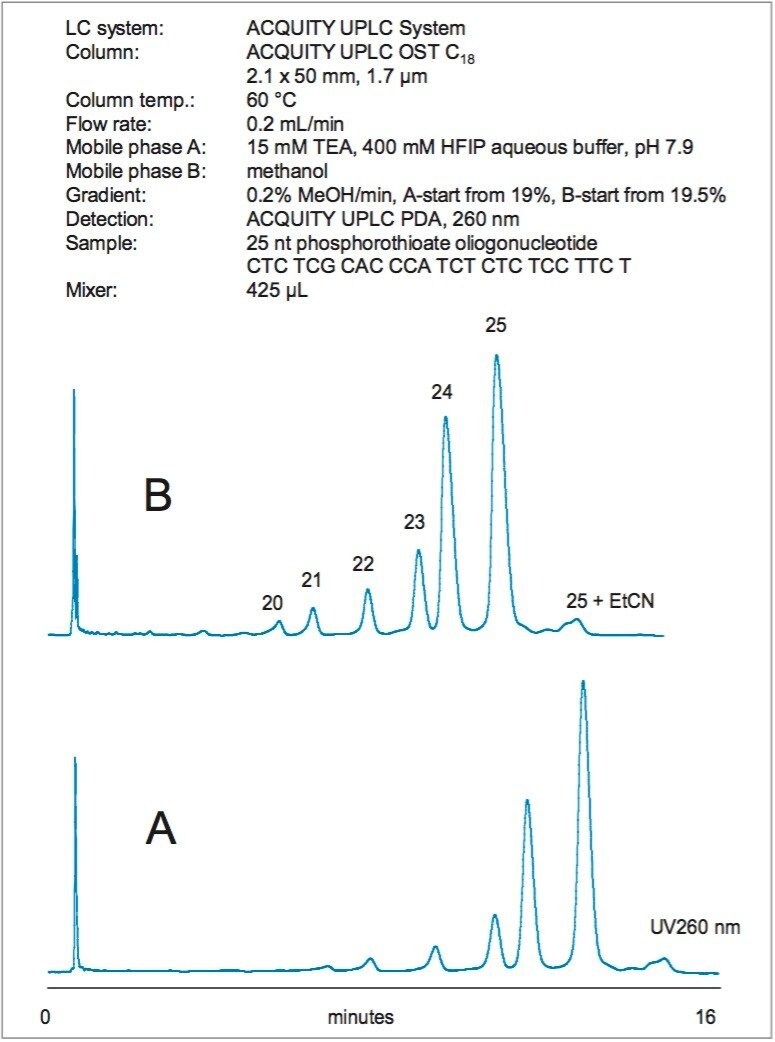
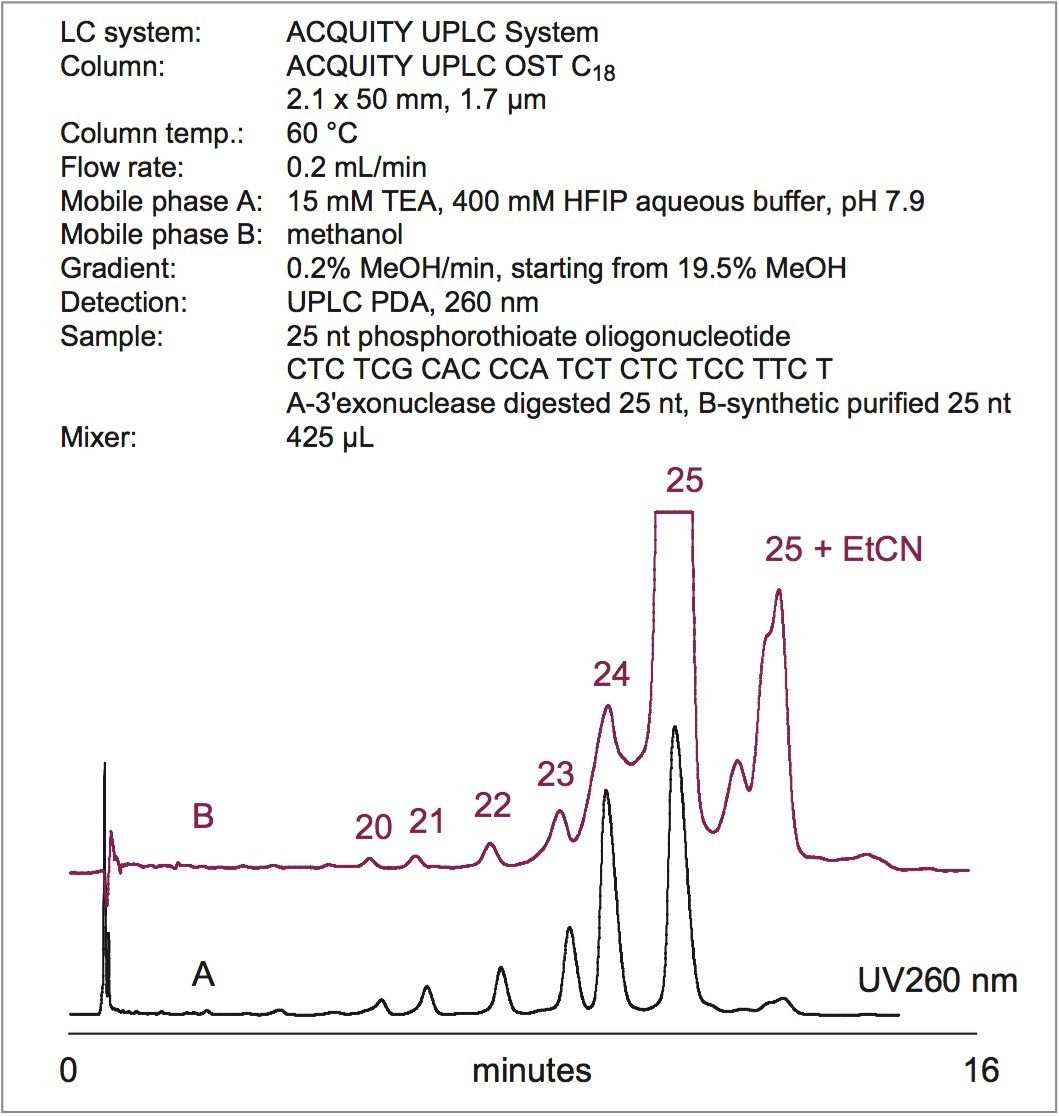
Figure 3 shows the analysis of a purified synthetic 25 nt phosphorothioate oligonucleotide. Interestingly, the failed synthesis by-products correspond to 3'-truncated parent oligonucleotide fragments. N+x peak (cyanoethyl protection group adduct; EtCN) was resolved from the target compound.
The ACQUITY UPLC System with Oligonucleotide Separation Technology Columns enable high-resolution, high-throughput analysis of phosphorothioate oligonucleotides in a single method. The development of fast analytical methods for native and modified oligonucleotides can be achieved quickly, increasing the overall operational efficiency of a laboratory. UPLC technology will increase the productivity of any laboratory developing LC and LC-MS methods and performing analysis of oligonucleotides.
720002405, November 2016